Open Access Article
Open Access ArticleC-Terminal modification of polytheonamide B uncouples its dual functions in MCF-7 cancer cells†
Yun-Wei
Xue
,
Kensuke
Miura
,
Hiroaki
Itoh
 and
Masayuki
Inoue
and
Masayuki
Inoue
 *
*
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan. E-mail: inoue@mol.f.u-tokyo.ac.jp
First published on 6th March 2023
Abstract
Polytheonamide B (1) is an exceptionally large peptide that forms a transmembrane ion channel. The potent cytotoxicity of 1 against MCF-7 cancer cells originates from its two ion transport functions. Compound 1 depolarizes the plasma membrane and neutralizes acidic lysosomes. Here, we describe how we uncoupled these functions by designing and synthesizing new analogues of 1.
Polytheonamide B (1, Scheme 1, Mw = 5030 Da) is a highly cytotoxic natural product isolated from the marine sponge Theonella swinhoei.1 The 48-mer amino-acid sequence of 1 possesses a D,L-alternating main chain and highly functionalized side chains, and is capped by a hydrophobic N-terminal 5,5-dimethyl-2-oxohexanoate group (Ncap). The hydrophobicity of the residues gradually decreases from the Ncap to residue-48, which is apparent from the non-polar and polar functional groups of each side chain.2 These unusual structural features together confer 1 with a unique molecular function. Compound 1 inserts from the N-terminus in a unidirectional manner into a lipid bilayer3,4 and forms a tubular β6.3-helix with a length of 4.5 nm and a pore size of 0.4 nm in diameter.5,6 The nanotube functions as a transmembrane ion channel with selectivity for monovalent cations (e.g., H+, Na+, K+, and Cs+). The potent cytotoxicity of 1 against cancer cells was originally considered to derive from the ion channel formation in the plasma membrane and the resulting disruption of the ion balance between the cell interior and exterior.
 | ||
| Scheme 1 Chemical structure of polytheonamide B (1) and its conversion to its C-terminal modified analogues 2 and 3. PyBOP = (benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate. Three-dimensional structure of β6.3-helix formed by 1 (PDB ID: 2RQO) is also displayed. | ||
A full solid-phase total synthesis of 1 was achieved in 2018, thereby enabling an efficient supply of 1 and its chemical probes.7 Investigation of the behavior in MCF-7 human breast cancer cells using synthetic peptides revealed an unprecedented mode of action of 1. Compound 1 not only induces free cation transport across the plasma membrane, but also diminishes the pH gradient in the lysosomal membrane after its endocytic internalization into the cells. These dual functions ultimately result in the apoptotic death of MCF-7 cells.
As the unusual biological activity of 1 is programmed by its exceptionally complex and large structure, molecular editing of 1 must perturbate its intrinsic cellular functions.8–10 Herein, we report the uncoupling of the dual actions of 1 on the plasma and lysosomal membranes by synthetically incorporating a tertiary amine at the C-terminus of 1.11–15 The present results thus provide the first valuable information for controlling the subcellular ion balance of cancer cells using the structure of 1.
The cytosol and extracellular environment are mostly neutral, whereas lysosomes can reach pH values as low as 4.5–4.7 to maintain the activities of encapsulated hydrolytic enzymes.16,17 Thus, the ion channel of 1 should mainly transport Na+ and K+ across the plasma membrane, and conduct H+ through the lysosomal membrane. Mechanistically, alkali cations pass through the water-filled tube of the folded 1,18 while proton conduction occurs by rearrangement of the hydrogen-bonded network of the water molecules, known as the Grotthuss proton hopping mechanism.19 We hypothesized that we could attenuate the original function of 1 in lysosomes by taking advantage of the distinct proton transfer mechanism.20 Accordingly, we designed two new analogues, 2 and 3, with dimethyl and diisopropyl amine moieties,21–24 respectively, at the C-terminus of 1 (Scheme 1). We assumed that the C-terminal-protonated amine would inhibit proton hopping across the lysosomal membrane by fixing the hydrogen-bonded network of the water molecules inside the tube.25,26 Although the C-terminal polarity and charge of 2 and 3 differ from that of 1, analogues 2 and 3 were expected to unidirectionally insert into the plasma membrane from the hydrophobic and unperturbed N-termini, similarly to 1. Furthermore, the C-terminal amines of 2 and 3 should be exposed to the acidic inner leaflet of the lysosomal membrane because lysosomes form via internalization of the plasma membrane.27
Polytheonamide B (1) was synthesized by solid-phase total synthesis and derivatized into the designed tertiary amine analogues 2 and 3via selective amidation at the C-terminal carboxylic acid (Scheme 1). To minimize epimerization at the Cα position of residue-48, PyBOP28 and HOAt29 were adopted as highly reactive condensation reagents and 2,4,6-collidine was used as a mild base. Under these conditions, N,N-dimethyl-1,4-butanediamine 4 or N,N-diisopropyl-1,4-butanediamine 5 were successfully coupled with 1 without the side-chain protective groups. Subsequent high-performance liquid chromatography purification provided 2 and 3 in 34% and 70% yields, respectively.
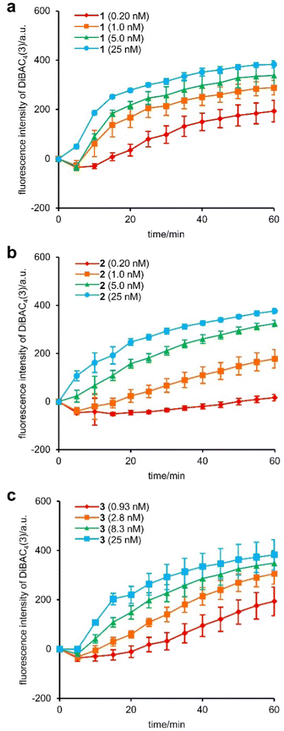
First, we compared the effects of 1, 2, and 3 on the plasma membrane of MCF-7 cells.30 Depolarization of the plasma membrane was assessed using DiBAC4(3).31 In this assay, when the ion channel insertion induces Na+/K+ exchange and decreases the potential across the plasma membrane, anionic DiBAC4(3) enters the cell and emits fluorescence by binding to intracellular proteins. Upon the addition of various concentrations of 1, 2, and 3, the fluorescence intensity of DiBAC4(3) increased in a time-dependent manner and reached a plateau within 1 h (Fig. 1a–c). The results corroborated the facile eradication of the plasma membrane potential by 1–3. To quantify the 50% effective concentrations (EC50) of the assay, 5-fold (0.20–25 nM for 1 and 2) or 3-fold (0.93–25 nM for 3) serial dilutions were applied. The EC50 values of 1, 2, and 3 were determined to be 1.1 nM, 2.2 nM, and 2.0 nM, respectively (Fig. S1, ESI†). Interestingly, the depolarization activity of 2 and 3 was comparable, indicating a negligible effect of the size difference of their tertiary amine moieties. On the other hand, 2 and 3 showed 2-fold weaker activities compared with 1. Hence, replacement of the negatively charged C-terminal carboxylate of 1 with the positively charged protonated amine of 2 and 3 decreased the Na+/K+ conductance, presumably due to a repulsive interaction between the ammonium and alkali cations. This inhibitory effect by the tertiary amine, however, was only modest.
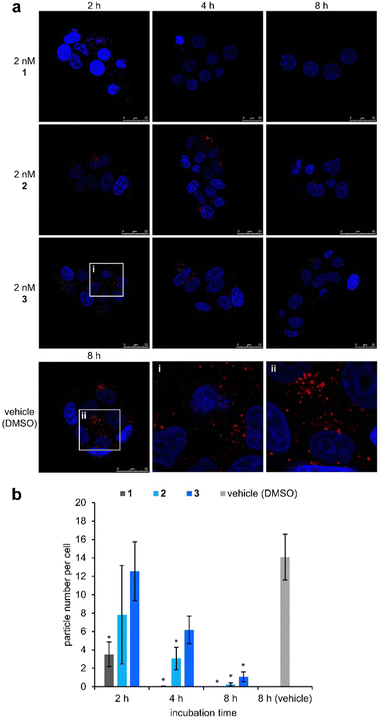
We next assessed the activities of 1, 2, and 3 against acidic lysosomes of MCF-7 cells. The lysosomal pH change was evaluated using a pH-sensitive fluorescent lysosomal marker, LysoTracker Red DND-99. The tertiary amine of LysoTracker is readily protonated in acidic media and therefore localized to acidic lysosomes. When an ion channel neutralizes the lysosome, the LysoTracker is liberated from the lysosome, which results in a decrease of the red fluorescence. In this assay, 1, 2, and 3 (2 nM) were applied to MCF-7 cells together with LysoTracker, and incubated for 2 h, 4 h, and 8 h (Fig. 2a). While the red fluorescence intensity was unchanged in the vehicle, it became negligible after 8 h treatment with 1, 2, and 3, corroborating the pH cancelling effects of these compounds. The neutralization efficiency of 2 and 3, however, was markedly decreased compared with that of 1. Although the addition of 1 led to a significant disappearance of the fluorescence within 2 h, the fluorescence signals were observed even after 4 h incubation in the cases of 2 and 3. To clarify the time-dependent activities of 1, 2, and 3, the numbers of LysoTracker-stained particles were calculated by image analysis (Fig. 2b and Fig. S2–S5, ESI†). The lysosomal pH gradient was eradicated by 2 and 3 over 8 h, but in a significantly slower manner compared with 1. The data obtained here strongly suggest that the C-terminal protonated tertiary amines of 2 and 3 attenuated the proton conductance by altering the hydrogen-bonded network of the water-filled transmembrane tube. Moreover, 3 was determined to be approximately 2-fold less active than 2. Therefore, the diisopropyl amine moiety of 3 more effectively decelerated the neutralization than the dimethyl amine moiety of 2, presumably because the bulkier amine of 3 more strongly hindered the proton exchange by the water molecules by locating itself at the entrance of the ion channel.
Finally, we investigated the cytotoxicity of 1, 2, and 3 against MCF-7 cells using a sulforhodamine B assay.32,33 The 50% inhibitory concentrations (IC50) were determined after 48 h incubation (Table 1 and Fig. S6, ESI†). Despite their distinct rates in decreasing the lysosomal proton gradient, 1, 2, and 3 all displayed comparable IC50 values (5.9 nM for 1, 5.0 nM for 2, 4.5 nM for 3). The consistent cytotoxicities of 1, 2, and 3 are likely attributable to the longer incubation times required for these experiments. Because depolarization of the plasma membrane and neutralization of the lysosomes were completed within 1 h and 8 h, respectively, 1, 2, and 3 ultimately displayed similar potent cytotoxic effects within 48 h.
In summary, we designed and synthesized new polytheonamide B analogues 2 and 3 with C-terminal dimethyl and diisopropyl amine structures, respectively. First, compounds 2 and 3 diminished the plasma membrane potential of MCF-7 cells, similarly to 1. Second, 2 and 3 were internalized and localized to acidic lysosomes, where they transport protons at a slower rate compared with 1. Thus, we successfully uncoupled these two original functions of 1 by simple molecular editing at the terminus of the extremely large molecule. Moreover, the different behaviors of 2 and 3 in lysosomes revealed the importance of the bulkiness of the tertiary amine for effectively decelerating the pH neutralization. Despite the disparate rates of 1, 2, and 3, neutralization was completed within 8 h. Thus, after 48 h incubation, 1, 2, and 3 all exhibited equipotent cytotoxicities. Overall, the present results offer new chemical insights for rationally modulating the pH homeostasis of cancer cells without changing the potent cytotoxicity of 1. More precise control of the ion balance of various cells and organelles will lead to the development of new anticancer drug seeds and biological tools based on natural product structures.34,35
This research was financially supported by JSPS with Grants-in-Aid for Scientific Research (S) (JP17H06110, JP22H04970) to M. I., and for Scientific Research (C) (JSPS, JP21K05286) to H. I. A fellowship from MEXT (WISE Program) to K. M. is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Hamada, S. Matsunaga, G. Yano and N. Fusetani, J. Am. Chem. Soc., 2005, 127, 110–118 CrossRef CAS PubMed.
- M. F. Freeman, M. J. Helf, A. Bhushan, B. I. Morinaka and J. Piel, Nat. Chem., 2017, 9, 387–395 CrossRef CAS PubMed.
- M. Iwamoto, H. Shimizu, I. Muramatsu and S. Oiki, FEBS Lett., 2010, 584, 3995–3999 CrossRef CAS PubMed.
- M. Kalathingal, T. Sumikama, S. Oiki and S. Saito, Biophys. J., 2021, 120, 4786–4797 CrossRef CAS PubMed.
- T. Hamada, S. Matsunaga, M. Fujiwara, K. Fujita, H. Hirota, R. Schmucki, P. Güntert and N. Fusetani, J. Am. Chem. Soc., 2010, 132, 12941–12945 CrossRef CAS PubMed.
- T. Mori, H. Kokubo, S. Oiki and Y. Okamoto, Mol. Simul., 2011, 37, 975–985 CrossRef CAS.
- A. Hayata, H. Itoh and M. Inoue, J. Am. Chem. Soc., 2018, 140, 10602–10611 CrossRef CAS PubMed.
- A. M. Szpilman and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 9592–9628 CrossRef CAS PubMed.
- H. Itoh and M. Inoue, Chem. Rev., 2019, 119, 10002–10031 CrossRef CAS PubMed.
- Z.-C. Wu and D. L. Boger, Nat. Prod. Rep., 2020, 37, 1511–1531 RSC.
- For previous studies of modulation of the original activities of 1 by molecular editing, see ref. 11–14: S. Matsuoka, N. Shinohara, T. Takahashi, M. Iida and M. Inoue, Angew. Chem., Int. Ed., 2011, 50, 4879–4883 CrossRef CAS PubMed.
- N. Shinohara, H. Itoh, S. Matsuoka and M. Inoue, ChemMedChem, 2012, 7, 1770–1773 CrossRef CAS PubMed.
- H. Itoh, S. Matsuoka, M. Kreir and M. Inoue, J. Am. Chem. Soc., 2012, 134, 14011–14018 CrossRef CAS PubMed.
- A. Hayata, H. Itoh, S. Matsutaka and M. Inoue, Chem. – Eur. J., 2016, 22, 3370–3377 CrossRef CAS PubMed.
- For an account, see: H. Itoh and M. Inoue, Acc. Chem. Res., 2013, 46, 1567–1578 CrossRef CAS PubMed.
- J. R. Casey, S. Grinstein and J. Orlowski, Nat. Rev. Mol. Cell Biol., 2010, 11, 50–61 CrossRef CAS PubMed.
- J. A. Mindell, Annu. Rev. Physiol., 2012, 74, 69–86 CrossRef CAS PubMed.
- M. Iwamoto, S. Matsunaga and S. Oiki, Sci. Rep., 2014, 4, 3636 CrossRef PubMed.
- Y. Matsuki, M. Iwamoto, K. Mita, K. Shigemi, S. Matsunaga and S. Oiki, J. Am. Chem. Soc., 2016, 138, 4168–4177 CrossRef CAS PubMed.
- For a review on controlling ion channel functions by chemical modification, see: J.-Y. Chen and J.-L. Hou, Org. Chem. Front., 2018, 5, 1728–1736 RSC.
- The tertiary amines used here are protonated in plasma and lysosomal membranes: H. K. Hall Jr., J. Am. Chem. Soc., 1957, 79, 5441–5444 CrossRef.
- G. A. Woolley, A. S. I. Jaikaran, Z. Zhang and S. Peng, J. Am. Chem. Soc., 1995, 117, 4448–4454 CrossRef CAS.
- P. Reiß, L. Al-Momani and U. Koert, ChemBioChem, 2008, 9, 377–379 CrossRef PubMed.
- M. X. Macrae, S. Blake, M. Mayer and J. Yang, J. Am. Chem. Soc., 2010, 132, 1766–1767 CrossRef CAS PubMed.
- J. L. Thomaston, N. F. Polizzi, A. Konstantinidi, J. Wang, A. Kolocouris and W. F. DeGrado, J. Am. Chem. Soc., 2018, 140, 15219–15226 CrossRef CAS PubMed.
- L. C. Watkins, W. F. DeGrado and G. A. Voth, J. Am. Chem. Soc., 2020, 142, 17425–17433 CrossRef CAS PubMed.
- Z. Darwich, A. S. Klymchenko, D. Dujardin and Y. Mély, RSC Adv., 2014, 4, 8481–8488 RSC.
- J. Coste, D. Le-Nguyen and B. Castro, Tetrahedron Lett., 1990, 31, 205–208 CrossRef CAS.
- L. A. Carpino, J. Am. Chem. Soc., 1993, 115, 4397–4398 CrossRef CAS.
- We selected MCF-7 cells for this study because this cell line was determined to be highly susceptible toward 1 (ref. 7). We also reported the investigation of the intracellular behavior of the structurally related gramicidin A using the same cell line: Y.-W. Xue, H. Itoh, S. Dan and M. Inoue, Chem. Sci., 2022, 13, 7482–7491 RSC.
- T. Bräuner, D. F. Hülser and R. J. Strasser, Biochim. Biophys. Acta, 1984, 771, 208–216 CrossRef PubMed.
- V. Vichai and K. Kirtikara, Nat. Protoc., 2006, 1, 1112–1116 CrossRef CAS PubMed.
- We also evaluated the cytotoxicity of natural 1 and analogues 2 and 3 using MCF-10A immortalized human breast epithelial cell line as a model of normal cells. Cytotoxicities of 1, 2, and 3 against MCF-10A were similar to those against MCF-7. See the ESI† for details.
- A. Henninot, J. C. Collins and J. M. Nuss, J. Med. Chem., 2018, 61, 1382–1414 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc05915k |
| This journal is © The Royal Society of Chemistry 2023 |