Open Access Article
Open Access ArticleA high-sensitivity long-lifetime phosphorescent RIE additive to probe free volume-related phenomena in polymers†
Valentina Antonia
Dini‡
a,
Alessandro
Gradone‡
*ab,
Marco
Villa
 a,
Marc
Gingras
a,
Marc
Gingras
 c,
Maria Letizia
Focarete
ad,
Paola
Ceroni
c,
Maria Letizia
Focarete
ad,
Paola
Ceroni
 a,
Chiara
Gualandi
a,
Chiara
Gualandi
 *ade and
Giacomo
Bergamini
*ade and
Giacomo
Bergamini
 *a
*a
aDepartment of Chemistry “Giacomo Ciamician” and INSTM UdR of Bologna, University of Bologna, Via Selmi, 2, 40126, Bologna, Italy. E-mail: c.gualandi@unibo.it; giacomo.bergamini@unibo.it
bCNR Institute for microelectronics and microsystems, Via Gobetti 101, 40129, Bologna, Italy. E-mail: gradone@bo.imm.cnr.it
cAix Marseille Univ, CNRS, CINAM, Marseille, France
dHealth Sciences and Technologies – Interdepartmental Center for Industrial Research (HST-ICIR), Alma Mater Studiorum – Università di Bologna, 40064 Ozzano dell’Emilia, Bologna, Italy
eInterdepartmental Center for Industrial Research on Advanced Applications in Mechanical Engineering and Materials Technology, CIRI-MAM, University of Bologna, Viale Risorgimento, 2, 40136, Bologna, Italy
First published on 12th January 2023
Abstract
The photophysical behaviour of phosphorescent rigidification-induced emission (RIE) dyes is highly affected by their micro- and nanoenvironment. The lifetime measure of RIE dyes dispersed in polymers represents an effective approach to gain valuable information on polymer free volume and thus develop materials potentially able to self-monitor physical ageing and mechanical stresses.
Luminescent organic compounds in solid state have found countless applications in the most diverse fields of research, from LED systems1 to stimuli-responsive switches2 and chemosensors.3 This is due to their low cost, affordable scale-up and lower or absence of toxicity compared to heavy metal ion-based emitters and quantum dots. In some applications of polymeric materials, the possibility of observing variations at the nanometer scale may help in understanding slight changes in properties and nano-organization that could have drastic consequences on the material's bulk properties.4 In this context, combining luminescent organic compounds and polymers can be an excellent way to endow materials with the capability of autonomously monitoring their long-term stability under conditions that can change their physical properties before failure.
Aggregation-Induced Emission (AIE) molecules have bright luminescence when aggregating in a solid state by restriction of intramolecular motions (RIM).5 This family of molecules can display bright luminescence also in rigid environments, like in frozen solution or supramolecular structure, so the term rigidification induced emission (RIE) could also be employed. Incorporation into the rigid environment of a polymer matrix is an excellent strategy to inhibit the dye's non-radiative decay by RIM and, consequently, luminescence intensity and lifetime increase.6–9 This phenomenon can be smartly exploited to acquire information about polymer behaviour, such as thermal transitions, dissolution, crystallinity, phase separation, cracking, and self-healing.10,11 For instance, fluorescent AIE dyes have been proposed as probes to determine polymer glass transition temperature (Tg)9 and, recently, topology freezing transition temperature (Tv) in vitrimers.12 These findings encourage the use of AIE dyes as polymer additives to endow materials with new functionalities, but there is still much room for improvement.
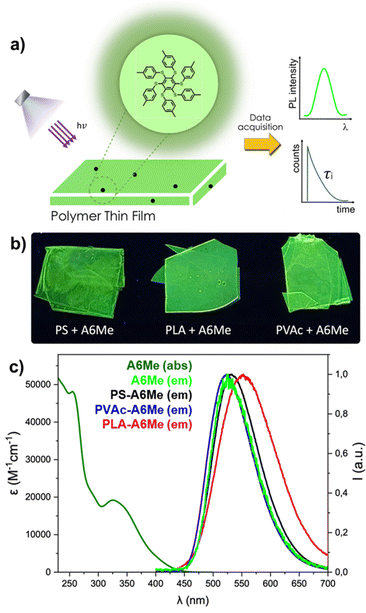
In the present work, a step forward is the exploitation of the phenomenon of phosphorescence that can bring several advantages over fluorescence since it is a spin-forbidden process, thus intrinsically slower than the spin-allowed fluorescence process. The long phosphorescence lifetime enables time-gated detection by recording the emission signal after a proper delay from the excitation. This measurement is more precise and allows the removal of undesired signals, like autofluorescence of the matrix or scattering of the excitation light. To explore the benefits of using phosphorescent RIE dyes as polymer additives, we have selected the AIEgen hexakis(4-methyl-1-phenylthio)benzene (A6Me), known as one of the most phosphorescent organic molecules in the solid state.13,14 It is non-emitting in solution at room temperature but in a rigid environment (i.e. in a frozen solution or a solid phase) it exhibits enhanced photophysical properties, with a very intense green phosphorescence and a long lifetime (Table S1, ESI†) as a consequence of RIM.15 A6Me is currently available on the market from several chemical suppliers, which incredibly increases its scientific impact on industry and academia.
A6Me was physically blended at a concentration of 2 wt%, with different polymer matrices of industrial relevance, i.e. polystyrene (PS), polyvinyl acetate (PVAc), and polylactic acid (PLA), by preparing films through solvent casting (see ESI†). The selected polymers are completely amorphous with Tg in the range of 40–120 °C, whose values (Table 1) are not affected by the addition of A6Me (Fig. S1, ESI†). The photophysical characterization is shown in Fig. 1, where the behaviour of the pristine dye is compared with the emissions obtained in the different unaged polymeric films. In the case of A6Me, it was previously demonstrated that the nature of the excited state has a significant charge transfer contributions,13 which in turn is notoriously strongly influenced by the polarity of the environment. The emission maximum of A6Me varies in the different polymers, probably due to different intermolecular interactions between the matrix and the dye. This interaction can be determined by the nature of polymer side groups, which can favor the “solubilization” of A6Me, depending on the overlap of the electronic clouds of the dye and the polymer. The effect of the matrix becomes more evident when recording lifetimes (Table 1). In the case of the complete immiscibility of dye and polymer, the lifetime is expected to be equal to that of the pristine dye in the solide state (i.e. 3 μs, Table S1, ESI†). However, when dispersed in the glassy polymers, A6Me always displays longer lifetimes, indicating a good dispersion of the molecule in the matrices and a possible effect of the matrix itself on the RIM mechanism.
The next focus of the work was to assess if lifetime measurements can provide information on local changes of polymer free volume. To this aim, we characterized the dye emission properties by measuring lifetime rather than the emission intensity since the latter brings many problems associated with its very nature. Indeed, the absolute value of emission intensity is massively affected by the optical properties of the polymer matrix (i.e. optical density, refractive index, sample thickness, presence of voids/microcracks that can induce waveguide effects or scattering, etc.) and the instrumentation parameters. Therefore, a reliable comparison between the emission of two different samples cannot be separated from the calculation of the emission quantum yield. Differently, lifetime, being an absolute measure, is not affected by the measuring conditions. In this study, lifetime observation represents the keystone for the application within the field of polymeric materials.
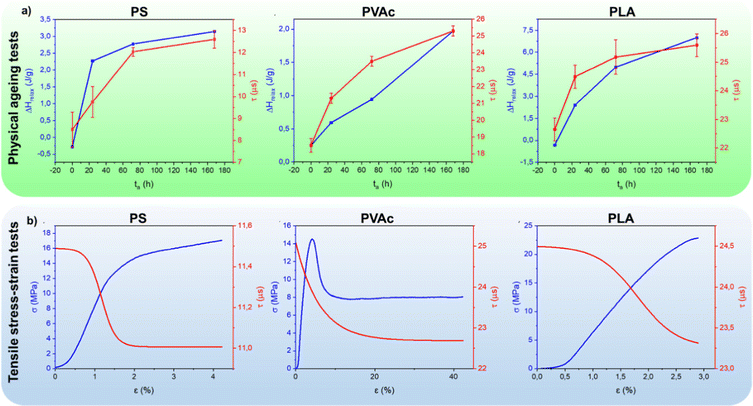
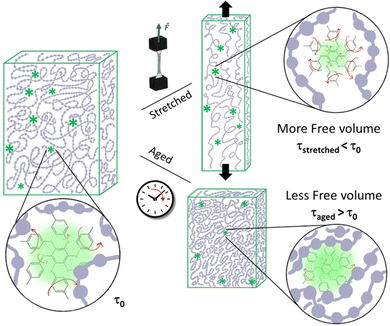
In glassy polymers, chain mobility decreases due to the free volume reduction to reach a thermodynamically stable state. This phenomenon, known as physical ageing, is due to small-scale chain relaxations that alter the local packing of the chains and cause volume contraction. The practical consequence of physical ageing is the increase of material brittleness, impairing long-term applications.4 Experimentally, ageing can be investigated by dilatometry, calorimetry and spectroscopic techniques such as positron annihilation lifetime spectroscopy (PALS), FTIR, and UV-Vis spectroscopy based on photochromic probes and labels.4 By using A6Me as a probe, we can take advantage of the long lifetime of the exited state and monitor lifetime variations in polymers subjected to accelerated physical ageing. The polymeric films were thermally annealed at a temperature below Tg for a specific time. For each polymer, Fig. 2a reports the lifetime of the dye and the enthalpy relaxation as a function of ageing time. As expected, the enthalpy relaxation strongly increases at short ageing times for the quenched samples, being far from the thermodynamic equilibrium, and continues to increase up to 7 days, albeit with a slower rate. Concomitantly, the dye's lifetime increases, following the same trend of enthalpy relaxation. Moreover, the radiative constant of the deactivation of the phosphorescent excited state increases upon ageing, while the non-radiative one decreases (Table S3, ESI†). This result demonstrates that the change in dye lifetime reflects the micro- and nanostructural changes of the glass occurring during thermal annealing, and can be related to the reduction of free volume, notoriously accompanied by material densification. In other words, the free volume available for A6Me rotational motions decreases with ageing time, thus limiting the non-radiative decay pathways of the excited state and enhancing the radiative deactivation.
Lifetime measurements were also carried out during tensile stress–strain tests. Fig. 2b reports for each polymer both the stress–strain curve and A6Me lifetime resulting from the fitting of experimental data recorded by positioning the optical fibre in front of the central area of the sample under tension (see Fig. S4 and Table S5 for the fitting procedure, ESI†). As expected, PS and PLA behave as rigid glassy polymers, showing a linear elastic region at low strain characterized by a high Young's module, followed, in the case of PLA, by a fragile fracture and, in the case of PS, by a short plastic deformation before breaking. Differently, PVAc behaves as a thermoplastic tough material, characterized by a lower Young's modulus in the elastic region and a strong yield followed by plastic deformation at constant stress before breaking (see mechanical data in Table S3 and Fig. S3, ESI†). A striking result is the evident reduction of A6Me lifetime during the elastic deformation in all host materials. In the case of PS and PLA, the lifetime linearly decreases in the elastic regime, reaching, in the case of PS, a constant value in the plastic regime. A similar behaviour, albeit with some differences, is observed in the lifetime of the dye dispersed in PVAc. Here, again, the fast decrease of A6Me lifetime occurs during matrix elastic deformation, but the decrement persists after yield, up to 20% of deformation, till reaching a constant value. The deformation mechanism of bulk isotropic linear glassy polymers in uniaxial tension has been the subject of intense experiments and numerical simulations aimed at correlating the mechanical behaviour with the deformation mechanism at the molecular level.16 Molecular dynamics simulations of a glassy amorphous polyethylene polymer under uniaxial tensile strength showed that the free volume increases in the elastic region while it reaches a constant value in the plastic deformation.17 This deduction nicely agrees with PALS analysis conducted on polycarbonate that experiences an increase in free volume when subjected to tensile deformation.18 Supported by these previous results, it is conceivable that the observed decrease in A6Me lifetime is due to the activation of dye motions in polymer sites where the free volume increases in the elastic regime. Mechanochromic polymeric systems, capable of monitoring deformation and stress to gain information on deformation mechanisms, are the subject of intensive research.19–25 The strain threshold for mechanoactivation can vary over a wide range, depending on the polymer under investigation and on the mechanoresponsive species, but, mechanochromism is generally achievable only after the yield point. This is the first observation of a luminescent probe sensitive to elastic deformation and represents a significant step forward and, at the same time, a starting point for future methodologies in this area.
In conclusion, we have developed auto-diagnostic materials where A6Me dye works as a probe of free volume changes in the surrounding polymeric matrices of three different polymers of industrial relevance. This all-organic dye was chosen to exploit its exalted and highly potent luminescence properties under the effect of an AIE-based process. In this work, we focused on correlating the dye's lifetime with the changes in the surrounding micro- and nano-organized environment. The dye lifetime is thus affected by small changes of free volume, occurring as a consequence of physical ageing, but also by molecular movements activated by mechanical stresses (Fig. 3).
Our methodological approach is based on: (i) an affordable, metal-free, relatively non-toxic organic dye with exalted phosphorescent properties; ii) a simple preparation method consisting in mixing a small amount of A6Me dye with the polymer without compromising its properties, and (ii) on lifetime detection that can be performed in situ and circumvents issues related to sample geometry and scattering effects, that may limit the reliability of emission intensity measurements. These results pave the way for further in-depth studies where phosphorescent AIEgens can be exploited to get helpful information on structural motions and nanostructural evolutions of polymers.
This work has been supported by the Italian Ministry of Education (MIUR), PRIN n° 20179BJNA2, Horizon 2020 Research and Innovation Programme “CHALLENGES” project n°861857, MG is grateful to the French National Agency for Research (ANR – program PRC) for the grant ANR-20-CE07-0031 “SulfurDance”. The project leading to this publication has received funding from the Excellence Initiative of Aix Marseille University – A*Midex, a French ‘‘Investissements d’Avenir’’ programme. MG also thanks the CNRS Program for International Scientific Collaborations (PICS no. 07573) with the Univ. of Bologna, Aix-Marseille Université, CINaM and CNRS France.
Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Gong and J. Kido, Adv. Mater., 2011, 23, 926 CrossRef CAS PubMed.
- T. Mutai, H. Satou and K. Araki, Nat. Mater., 2005, 4, 685 CrossRef CAS PubMed.
- Y. Yang, X. Su, C. N. Carroll and I. Aprahamian, Chem. Sci., 2012, 3, 610 RSC.
- D. Cangialosi, V. M. Boucher, A. Alegría and J. Colmenero, Soft Matter, 2013, 9, 8619 RSC.
- Z. Qiu, X. Liu, J. W. Y. Lam and B. Z. Tang, Macromol. Rapid Commun., 2019, 40, 1 CrossRef PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- G. Iasilli, A. Battisti, F. Tantussi, F. Fuso, M. Allegrini, G. Ruggeri and A. Pucci, Macromol. Chem. Phys., 2014, 215, 499 CrossRef CAS.
- T. Han, C. Gui, J. W. Y. Lam, M. Jiang, N. Xie, R. T. K. Kwok and B. Z. Tang, Macromolecules, 2017, 50, 5807 CrossRef CAS.
- S. Bao, Q. Wu, W. Qin, Q. Yu, J. Wang, G. Liang and B. Z. Tang, Polym. Chem., 2015, 6, 3537 RSC.
- S. Ge, E. Wang, J. Li and B. Z. Tang, Macromol. Rapid Commun., 2022, 43, 2200080 CrossRef CAS PubMed.
- P. Bosch, F. Catalina, T. Corrales and C. Peinado, Chem. – Eur. J., 2005, 11, 4314 CrossRef CAS PubMed.
- Y. Yang, S. Zhang, X. Zhang, L. Gao, Y. Wei and Y. Ji, Nat. Commun., 2019, 10, 1 CrossRef PubMed.
- G. Bergamini, A. Fermi, C. Botta, U. Giovanella, S. Di Motta, F. Negri, R. Peresutti, M. Gingras and P. Ceroni, J. Mater. Chem. C, 2013, 1, 2717 RSC.
- A. Fermi, G. Bergamini, R. Peresutti, E. Marchi, M. Roy, P. Ceroni and M. Gingras, Dyes Pigm., 2014, 110, 113 CrossRef CAS.
- M. Baroncini, G. Bergamini and P. Ceroni, Chem. Commun., 2017, 53, 2081 RSC.
- E. F. Oleinik, M. A. Mazo, I. A. Strel’nikov, S. N. Rudnev and O. B. Salamatina, Polym. Sci., Ser. A, 2018, 60(1), 1–49 CrossRef CAS.
- D. Hossain, M. A. Tschopp, D. K. Ward, J. L. Bouvard, P. Wang and M. F. Horstemeyer, Polymer, 2010, 51, 6071 CrossRef CAS.
- L. Xie, D. W. Gidley, H. A. Hristov and A. F. Yee, J. Polym. Sci., Part B: Polym. Phys., 1995, 33, 77 CrossRef CAS.
- H. Traeger, D. J. Kiebala, C. Weder and S. Schrettl, Macromol. Rapid Commun., 2021, 42, 1 CrossRef PubMed.
- F. Kempe, O. Brügner, H. Buchheit, S. N. Momm, F. Riehle, S. Hameury, M. Walter and M. Sommer, Angew. Chem., Int. Ed., 2018, 57, 997 CrossRef CAS PubMed.
- N. Willis-Fox, E. Rognin, T. A. Aljohani and R. Daly, Chem, 2018, 4, 2499 CAS.
- Q. Guo and X. Zhang, Composites, Part B, 2021, 227, 109434 CrossRef.
- G. Chen and W. Hong, Adv. Opt. Mater., 2020, 8, 1 CAS.
- F. Ciardelli, G. Ruggeri and A. Pucci, Chem. Soc. Rev., 2013, 42, 857 RSC.
- J. Li, C. Nagamani and J. S. Moore, Acc. Chem. Res., 2015, 48, 2181 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc05908h |
| ‡ The author contributes equally to the work described in this communication. |
| This journal is © The Royal Society of Chemistry 2023 |