Open Access Article
Open Access ArticleSelf-oxidation of cysteine to sulfinic acid in an engineered T67C myoglobin: structure and reactivity†
Wei
Dai‡
a,
Hong
Yuan‡
b,
Xiao-Juan
Wang
a,
Shu-Qin
Gao
c,
Xiangshi
Tan
 b and
Ying-Wu
Lin
b and
Ying-Wu
Lin
 *ac
*ac
aSchool of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China. E-mail: ywlin@usc.edu.cn
bDepartment of Chemistry & Institute of Biomedical Science, Fudan University, Shanghai 200433, China
cKey Lab of Protein Structure and Function of Universities in Hunan Province, University of South China, Hengyang 421001, China
First published on 15th February 2023
Abstract
Myoglobin (Mb) was found to undergo self-oxidation when a cysteine residue was engineered at position 67 in the heme distal site. Both the X-ray crystal structure and mass spectrum confirmed the formation of a sulfinic acid (Cys–SO2H). Moreover, the self-oxidation could be controlled during protein purification to yield the unmodified form (T67C Mb). Importantly, both T67C Mb and T67C Mb (Cys–SO2H) were able to be labeled by chemicals, which provided useful platforms to generate artificial proteins.
As a redox-active amino acid, cysteine (Cys) plays vital roles in supporting protein structure and regulating protein function via many oxidative post-translational modifications (PTMs).1–4 For example, in addition to forming disulfide bond, Cys residues are susceptible to oxidation by reactive oxygen/nitrogen/sulfur (RO/N/S) species, generating sulfenic/sulfinic acids (Cys–SOH/SO2H), S-nitrosothiol and persulfide species, etc., which mediate the redox signaling in biological systems.1,4
Heme proteins are a large class of metalloproteins and use an iron protoporphyrin IX (heme) as a cofactor, and in some of them the Cys residue is irreplaceable.5–7 In cytochrome P450 (CYP450), Cys acts as a proximal ligand for the heme cofactor to activate O2,8 and in human neuroglobin (Ngb) Cys46 and Cys55 form an intramolecular disulfide bond to regulate the ligand binding.9–12 Moreover, the third Cys120 in human Ngb may also regulate the intracellular levels of RO/N/S species.13 As an O2 carrier, human myoglobin (Mb) possesses a single Cys110 and efficient reduction of the Cys-thiyl radical was observed by electron transfer from glutathione, whereas other mammalian Mbs rarely have Cys residues.14
To investigate the effects of Cys on Mb (Fig. 1A), Hirota et al. introduced a Cys at various positions of sperm whale Mb on the protein surface or near the heme such as at position 96, and observed that heme reduction occurred under a CO atmosphere by intramolecular electron transfer.15 In previous studies, we also introduced a Cys close to the heme group.16,17 It was shown that a Cys introduced at position 42 (K42C mutation) was covalently-linked to the heme 4-vinyl group under reducing conditions in the presence of O2,16 whereas it did not happen for Cys introduced at position 46 with a long distance to the heme iron (Fig. 1A). Instead, Cys46 could form a disulfide bond with a Cys introduced nearby at position 49, 55 or 61, which regulates the protein structure and function.17,18
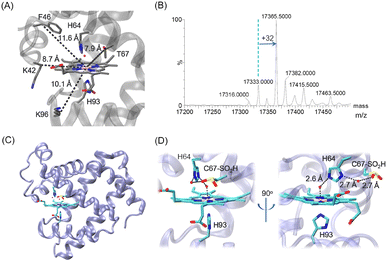 | ||
| Fig. 1 (A) X-ray structure of wild-type (WT) Mb (PDB code 1JP619) showing the heme coordination site (His64/His93) and surrounding residues, which were mutated to Cys in previous studies (K96C, K42C and F46C) and in this study (T67C), respectively, with the distances from the CB atom to the heme iron indicated. (B) ESI-MS spectrum of T67C Mb. (C and D) The X-ray structure of T67C Mb (Cys–SO2H) (PDB code 7XCF, this study), showing the overall structure (C) and the heme coordination structure (D), respectively. The H-bonding interactions are indicated by dashed lines. | ||
Inspired by this progress, we attempted to introduce a Cys close to the heme group at position 67 (Fig. 1A, ∼7.9 Å) by T67C mutation. The T67C Mb was expressed and purified from E. coli cells using a procedure similar to that for WT Mb. Unexpectedly, the mass spectra showed that the protein has several forms (Fig. 1B, and Fig. S1 for the multiple m/z peaks, ESI†). The observed mass of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333.0 ± 0.5 Da agreed with the calculated mass of T76C Mb (17
333.0 ± 0.5 Da agreed with the calculated mass of T76C Mb (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333.0 Da), whereas the major form showed a mass of 17
333.0 Da), whereas the major form showed a mass of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365.5 ± 0.5 Da, with an increase of ∼32 Da, which was likely due to the modification of Cys67 by the addition of two O atoms, as a result of oxidation during protein expression or purification.
365.5 ± 0.5 Da, with an increase of ∼32 Da, which was likely due to the modification of Cys67 by the addition of two O atoms, as a result of oxidation during protein expression or purification.
To provide direct evidence for the oxidation of Cys67, we crystallized the mutant and solved the X-ray crystal structure at a resolution of 1.70 Å (Table S1, ESI†). As shown in Fig. 1C and D, it revealed that Cys67 was oxidized to a sulfinic acid (Cys–SO2H), in agreement with the observation for the major form in the MS spectrum. The heme axial water formed a hydrogen (H)-bond with the distal His64, which is similar to that in WT Mb. Moreover, an additional water molecule was found to bridge the N-atom of His64 and an O-atom of Cys67–SO2H by H-binding interactions. The structural overlay also showed that the introduction and modification of Cys67–SO2H did not alter the overall structure of Mb, as well as for the secondary structure (Fig. S2, ESI†). This was further confirmed by the circular dichroism spectroscopy of T67C Mb (Cys–SO2H), with a proportion of α-helixes similar to that of WT Mb (Fig. S3, ESI†).
In consideration of the mechanism for the self-oxidation of Cys67, we speculated that several factors may contribute to the modification. First, catalytic iron species (Compound I, an oxoferryl heme π-cation radical, or Compound II, oxoferryl heme) may be generated by activating O2 upon binding to the heme iron, as proposed for other heme proteins such as CYP450, cytochrome c and Ngb mutants.20–23 Second, Cys at position 67 is close to the heme iron with a short distance of ∼8 Å (Fig. 1), which is prone to be modified. Moreover, the distance between the sulfur atom of Cys67 and the nitrogen atom of His64 is ∼5.5 Å. It has been reported that histidine may act as a proton acceptor, which decreases the pKa of surrounding Cys and makes it easier to be oxidized.24 In addition, it showed that the direct reaction of T67C Mb with H2O2 increased the proportion of forms with +32 Da and +48 Da (Fig. S4, ESI†), suggesting the oxidation of Cys67–SH to both sulfinic (Cys–SO2H) and sulfinate (Cys–SO3H) acids.
To explore the reactivity of T67C Mb (Cys–SO2H), we performed the reaction with diisopropyl azodicarboxylate (DIAD) (Fig. 2A). In a previous study, organic diazene compounds have been used for the detection of sulfinic acid, because the attack of a sulfinate on the electron-deficient nitrogen yields a stable sulfonamide.25 As shown in Fig. 2B by ESI-MS analysis, an increase in mass of 203 Da was observed for T67C Mb (Cys–SO2H) after the reaction with DIAD (202 Da), indicating the covalent attachment of DIAD to the Cys–SO2H group. Moreover, the result also showed that both the forms of Cys67–SH and Cys67–SO3H were involved in the reaction to produce the corresponding sulfonamides (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535.0 Da and 17
535.0 Da and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 583.5 Da, respectively), suggesting no specific modification of the Cys–SO2H group.
583.5 Da, respectively), suggesting no specific modification of the Cys–SO2H group.
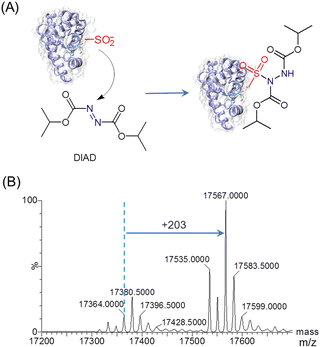 | ||
| Fig. 2 (A) Reactivity of T67C Mb (Cys–SO2H) toward diisopropyl azodicarboxylate (DIAD). (B) ESI-MS spectrum of T67C Mb (Cys–SO2H) after reaction with DIAD (3.0 eq.) for 9 h at 25 °C. | ||
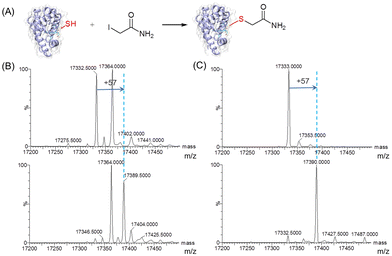
To further evaluate the reactivity of T67C Mb (Cys–SH&SO2H), we carried out site-selective protein-modification studies using iodoacetamide (IAM) as a labeling reagent (Fig. 3A).26 As shown in Fig. 3B, after the reaction with IAM, only the form of T67C Mb (Cys–SH) reacted with IAM, resulting in an increase of 57 Da, whereas T67C Mb (Cys–SO2H) retained unmodified. We noticed that after T67C Mb (Cys–SH&SO2H) was exposed to air for a few days, most Cys was self-oxidized to the form of Cys–SO2H, which may also occur for the crystallization. In order to obtain the unmodified form of T67C Mb (Cys–SH), we simplified the purification process by using only anion exchange chromatography, which reduces the time of the protein exposed to air. As shown in Fig. 3C (top), T67C Mb (Cys–SH) could be obtained with a high purity (the multiple m/z peaks were shown in Fig. S5, ESI†). The UV-Vis spectrum was similar to that of T67C Mb (Cys–SO2H) (Fig. S6, ESI†). The reaction of T67C Mb (Cys–SH) with IAM further showed that the protein was fully covalently modified by IAM (Fig. 3C, bottom). The oxidation of Cys67 may occur in the extracellular environment, whereas the intracellular reducing environment may protect it from oxidation, which might be different from the oxidative stress of the human DJ-1 protein.27 These results indicate that Cys67 is both oxidant-sensitive and reactive toward chemicals, which is expected to modulate the protein structure and function by covalent chemical modifications.
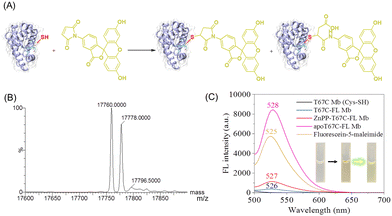
In addition, to explore the reactivity of Cys67 in T67C Mb (Cys–SH), we conjugated a fluorescein molecule to the protein by using fluorescein-5-maleimide (FL) as a labeling reagent (Fig. 4A).28 The mass spectra showed that two major products were formed (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760.0 and 17
760.0 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 778.0 Da), which suggests that FL was covalently linked to the protein, whereas the ring-opening product was formed by hydrolysis (Fig. 4B). The fluorescence spectra showed that T67C-FL Mb exhibited a very low intensity at 526 nm compared to that of the free FL at 525 nm, which suggests that an energy transfer occurred since FL was linked close to the heme group, as reported recently by Hayashi and co-workers.29
778.0 Da), which suggests that FL was covalently linked to the protein, whereas the ring-opening product was formed by hydrolysis (Fig. 4B). The fluorescence spectra showed that T67C-FL Mb exhibited a very low intensity at 526 nm compared to that of the free FL at 525 nm, which suggests that an energy transfer occurred since FL was linked close to the heme group, as reported recently by Hayashi and co-workers.29
To provide evidence for the energy transfer, we prepared apo-T67C-FL Mb by removing the heme group. The fluorescence spectrum of apo-T67C-FL Mb showed the characteristic emission at 528 nm, with the intensity higher than that of free FL. Upon chemically modifying the maleimide, the photoinduced electron transfer was interrupted, which restores the fluorescence.30 Moreover, we reconstituted the apo-T67C-FL Mb with a heme analog, zinc protoporphyrin (ZnPP), as confirmed by the UV-Vis spectra (Fig. S7, ESI†).31–33 The fluorescence spectra further showed that ZnPP-T67C-FL Mb exhibited an emission at 527 nm, with an intensity 3-fold higher than that of T67C-FL Mb (Fig. 4C). These observations suggest that protoporphyrins with different metal centers may quench the fluorescence of the conjugated molecule to a different degree, which might be used as a probe to detect the interactions between the conjugated molecule and the protein cofactor.
In summary, we found that after introducing a redox-active Cys to the heme distal position 67 of Mb, it underwent self-oxidation, and majorly formed a sulfinic acid (Cys–SO2H), which was confirmed by the X-ray crystal structure and mass spectrum analysis. This modification did not alter the protein structure, which could also be controlled by simplifying the protein purification to yield the unmodified form, T67C Mb (Cys–SH). Importantly, protein reactivity showed that T67C Mb (Cys–SO2H) could be labeled by DIAD, whereas T67C Mb (Cys–SH) could be labeled by DIAD, IAM, and fluorescein molecules, which provided useful platforms to generate artificial proteins for studying the structure and function of heme proteins,34,35 such as the energy transfer between a conjugated molecule and the protein cofactor.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully thank Prof. S. G. Sligar and Prof. Y. Lu of the University of Illinois at Urbana-Champaign, USA, for the gift of the Mb gene. X-ray diffraction data were collected at Shanghai Synchrotron Radiation Facility, China. This work was supported by the National Natural Science Foundation of China (32171270 and 21977017).References
- C. E. Paulsen and K. S. Carroll, Chem. Rev., 2013, 113, 4633–4679 CrossRef CAS PubMed.
- J. M. Chalker, G. J. L. Bernardes, Y. A. Lin and B. G. Davis, Chem. – Asian J., 2009, 4, 630–640 CrossRef CAS PubMed.
- L. J. Alcock, M. Langini, K. Stuhler, M. Remke, M. V. Perkins, G. J. L. Bernardes and J. M. Chalker, ChemBioChem, 2020, 21, 1329–1334 CrossRef CAS.
- R. B. Ferreira, L. Fu, Y. Jung, J. Yang and K. S. Carroll, Nat. Commun., 2022, 13, 5522 CrossRef CAS PubMed.
- T. L. Poulos, Chem. Rev., 2014, 114, 3919–3962 CrossRef CAS PubMed.
- J. Liu, S. Chakraborty, P. Hosseinzadeh, Y. Yu, S. Tian, I. Petrik, A. Bhagi and Y. Lu, Chem. Rev., 2014, 114, 4366–4469 CrossRef CAS PubMed.
- Y.-W. Lin, Arch. Biochem. Biophys., 2018, 641, 1–30 CrossRef CAS PubMed.
- I. G. Denisov, T. M. Makris, S. G. Sligar and I. Schlichting, Chem. Rev., 2005, 105, 2253–2277 CrossRef CAS PubMed.
- T. Burmester, B. Weich, S. Reinhardt and T. Hankeln, Nature, 2000, 407, 520–523 CrossRef CAS PubMed.
- B. G. Guimaraes, D. Hamdane, C. Lechauve, M. C. Marden and B. Golinelli-Pimpaneau, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2014, 70, 1005–1014 CrossRef CAS PubMed.
- S.-Q. Gao, H. Yuan, X.-C. Liu, L. Li, X. Tan, G.-B. Wen and Y.-W. Lin, Proteins, 2022, 90, 1152–1158 CrossRef CAS PubMed.
- L. Chen, H. Yuan, X.-J. Wang, L. Li, X. Tan and Y.-W. Lin, ChemBioChem, 2022, 23, e202200531 CAS.
- P. Ascenzi, A. di Masi, L. Leboffe, M. Fiocchetti, M. T. Nuzzo, M. Brunori and M. Marino, Mol. Aspects Med., 2016, 52, 1–48 CrossRef CAS PubMed.
- S. Nagao, O. Asami, H. Yasui and S. Hirota, Biochim. Biophys. Acta, 2011, 1814, 480–486 CrossRef CAS PubMed.
- S. Hirota, K. Azuma, M. Fukuba, S. Kuroiwa and N. Funasaki, Biochemistry, 2005, 44, 10322–10327 CrossRef CAS PubMed.
- H.-M. Cheng, H. Yuan, X.-J. Wang, J.-K. Xu, S.-Q. Gao, G.-B. Wen, X. Tan and Y.-W. Lin, J. Inorg. Biochem., 2018, 182, 141–149 CrossRef CAS PubMed.
- L.-J. Sun, H. Yuan, L. Yu, S.-Q. Gao, G.-B. Wen, X. Tan and Y.-W. Lin, Chem. Commun., 2022, 58, 5885–5888 RSC.
- L.-J. Sun, H. Yuan, J.-K. Xu, J. Luo, J.-J. Lang, G.-B. Wen, X. Tan and Y.-W. Lin, Biochemistry, 2023, 62, 369–377 CrossRef CAS PubMed.
- P. Urayama, G. N. Phillips, Jr. and S. M. Gruner, Structure, 2002, 10, 51–60 CrossRef CAS PubMed.
- J. Rittle and M. T. Green, Science, 2010, 330, 933–937 CrossRef CAS PubMed.
- P. C. E. Moody and E. L. Raven, Acc. Chem. Res., 2018, 51, 427–435 CrossRef CAS PubMed.
- Z. Wang, Y. Ando, A. D. Nugraheni, C. Ren, S. Nagao and S. Hirota, Mol. BioSyst., 2014, 10, 3130–3137 RSC.
- H.-X. Liu, L. Li, B. He, S.-Q. Gao, G.-B. Wen and Y.-W. Lin, Dalton Trans., 2018, 47, 10847–10852 RSC.
- D. Garrido Ruiz, A. Sandoval-Perez, A. V. Rangarajan, E. L. Gunderson and M. P. Jacobson, Biochemistry, 2022, 61, 2165–2176 CrossRef CAS PubMed.
- S. Akter, L. Fu, Y. Jung, M. L. Conte, J. R. Lawson, W. T. Lowther, R. Sun, K. Liu, J. Yang and K. S. Carroll, Nat. Chem. Biol., 2018, 14, 995–1004 CrossRef CAS PubMed.
- Y. H. Kuo, A. M. Konopko, N. B. Borotto, J. D. Majmudar, S. E. Haynes and B. R. Martin, ChemBioChem, 2017, 18, 2028–2032 CrossRef CAS PubMed.
- J. Lin, J. Prahlad and M. A. Wilson, Biochemistry, 2012, 51, 3799–3807 CrossRef CAS PubMed.
- J. S. Nanda and J. R. Lorsch, Methods Enzymol., 2014, 536, 87–94 CAS.
- J. W. Soon, K. Oohora and T. Hayashi, RSC Adv., 2022, 12, 28519–28524 RSC.
- K. Renault, J. W. Fredy, P. Y. Renard and C. Sabot, Bioconjug. Chem., 2018, 29, 2497–2513 CrossRef CAS PubMed.
- S. V. Lepeshkevich, M. V. Parkhats, A. S. Stasheuski, V. V. Britikov, E. S. Jarnikova, S. A. Usanov and B. M. Dzhagarov, J. Phys. Chem. A, 2014, 118, 1864–1878 CrossRef CAS PubMed.
- Z.-H. Shi, K.-J. Du, B. He, S.-Q. Gao, G.-B. Wen and Y.-W. Lin, Inorg. Chem. Front., 2017, 4, 2033–2036 RSC.
- A. Tangar, V. Derrien, R. Lei, M. J. Santiago Estevez, P. Sebban, S. Bernad and J. Miksovska, Metallomics, 2019, 11, 906–913 CrossRef CAS PubMed.
- Y.-W. Lin, Coord. Chem. Rev., 2017, 36(1), 1–27 CrossRef.
- Y.-W. Lin, Coord. Chem. Rev., 2021, 434, 213774 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, ESI-MS, UV-Vis, CD spectra, and crystallography data. See DOI: https://doi.org/10.1039/d3cb00007a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |