Open Access Article
Open Access ArticleImmunotoxicity of metal and metal oxide nanoparticles: from toxic mechanisms to metabolism and outcomes
Jiaming
Bi†
a,
Chuzi
Mo†
a,
Siwei
Li
a,
Mingshu
Huang
 b,
Yunhe
Lin
a,
Peiyan
Yuan
a,
Zhongjun
Liu
*a,
Bo
Jia
b,
Yunhe
Lin
a,
Peiyan
Yuan
a,
Zhongjun
Liu
*a,
Bo
Jia
 *b and
Shuaimei
Xu
*b and
Shuaimei
Xu
 *a
*a
aDepartment of Endodontics, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, Guangdong, China. E-mail: xushuaimei@smu.edu.cn; liuzhongjundr@smu.edu.cn
bDepartment of Oral and Maxillofacial Surgery, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, Guangdong, China. E-mail: dentist-jia@163.com
First published on 18th April 2023
Abstract
The influence of metal and metal oxide nanomaterials on various fields since their discovery has been remarkable. They have unique properties, and therefore, have been employed in specific applications, including biomedicine. However, their potential health risks cannot be ignored. Several studies have shown that exposure to metal and metal oxide nanoparticles can lead to immunotoxicity. Different types of metals and metal oxide nanoparticles may have a negative impact on the immune system through various mechanisms, such as inflammation, oxidative stress, autophagy, and apoptosis. As an essential factor in determining the function and fate of immune cells, immunometabolism may also be an essential target for these nanoparticles to exert immunotoxic effects in vivo. In addition, the biodegradation and metabolic outcomes of metal and metal oxide nanoparticles are also important considerations in assessing their immunotoxic effects. Herein, we focus on the cellular mechanism of the immunotoxic effects and toxic effects of different types of metal and metal oxide nanoparticles, as well as the metabolism and outcomes of these nanoparticles in vivo. Also, we discuss the relationship between the possible regulatory effect of nanoparticles on immunometabolism and their immunotoxic effects. Finally, we present perspectives on the future research and development direction of metal and metal oxide nanomaterials to promote scientific research on the health risks of nanomaterials and reduce their adverse effects on human health.
1. Introduction
In recent decades, nanomaterials have been widely applied in industrial manufacturing and the food and medicine sectors, promoting the rapid development of related technologies. However, although nanomaterials have become increasingly popular, their toxic effects on humans are not clearly understood. In the field of biomedicine, metal and metal oxide NPs (including Ag, Au, ZnO, CuO, and CeO2 NPs) are widely used as drug delivery systems and diagnostic, therapeutic and imaging systems due to their unique physical and chemical properties. However, the immunotoxicity caused when these NPs interact with immune cells has raised concerns about their safety. Numerous studies have shown that metal and metal oxide NPs pose a risk to the human body, inducing a series of reactions through its defence system.1 The immune response refers to the defensive response of the body to foreign components or mutated autologous components, which can be divided into non-specific and specific immune responses. When metal and metal oxide NPs enter the human body, they are recognised as ‘foreign’ substances by the immune system and trigger a series of immune responses. The relevant tissue- and organ-level regulations enable the body to respond quickly and adapt to the NP stimulus within a short period.2 Although this can lead to positive immune responses (e.g., NPs can participate in and coordinate the immune response as a vaccine adjuvant), in some cases, negative immune responses occur3 (i.e., immunotoxicity).The field of immunometabolism has developed rapidly in recent years, revealing the contribution of biochemistry to the development, fate, and behaviour of immune cells. The manipulation of specific components of the immunometabolism cycle (such as TORC1, TORC2, PTEN, AMPK, and PI3K) by genetic or pharmacological methods can affect the energy metabolism in immune cells, thereby regulating cell behaviour and function.4 Therefore, metal and metal oxide NPs may also affect the differentiation and functions of immune cells by affecting immunometabolism, which may also contribute to the immunotoxicity caused by these NPs.
The toxic effects of metal and metal oxide NPs mainly depend on their physicochemical properties, such as composition, size, geometry, surface charge, and coating material.5 After administration, the physical and chemical properties of NPs are altered. NPs can aggregate, agglomerate, dissolve or degrade, and eventually excreted through the metabolism-related tissues and organs.1 During this process, NPs and their degradation products may affect the roles and functions of immune cells and metabolism-related tissues and organs. Therefore, a complete understanding of their effects on the immune system, especially their immunotoxicity and fate in vivo, are essential for developing better NPs with good biosafety.
Previous reviews mainly focused on the immunotoxicity of specific metal or metal oxide NPs or the toxic effects observed in particular tissues. Thus, a systematic summary of the possible mechanisms of the immunotoxicity caused by these NPs is lacking. Different types of metal and metal oxide NPs have different structures and properties and may exhibit different cellular mechanisms of immunotoxicity. To provide a comprehensive and systematic review, herein, we introduce the structure, properties, and applications of different types of metal and metal oxide NPs. Subsequently, we summarise and delineate the immunotoxic effects of metal and metal oxide NPs based on their toxicity mechanisms. In addition, we describe the metabolism and outcomes of these NPs in vivo. Finally, we discuss the possible effects of different physicochemical properties and experimental conditions on the degradation, metabolism, and excretion of metal and metal oxide NPs. The relationship between the regulatory effect of nanoparticles on immunometabolism and their toxic effects is also discussed to understand the immunological properties of these NPs and offer additional prospects for designing safer NPs.
2. Classification, characteristics and applications of metal and metal oxide NPs
NPs are tiny particles with a size in the range of 1 to 100 nm in a particular dimension within three-dimensional space. ‘Nanometals’, which are metallic materials manufactured via nanotechnology, have a nanoscale structure and contain nanoparticle impurities.6 Using nanotechnology, it is possible to control the composition and microstructure of metallic materials with extreme precision and intricacy during their production. Consequently, the mechanical and functional properties of metals have been improved by leaps and bounds. NPs can be mono-metallic or consist of metal alloys or metal oxides (such as Au NPs, Cu–Ag NPs, and CuO NPs, respectively). Metallic materials can be classified into different groups, which may have different physical and chemical properties and characteristics. Therefore, the corresponding metal and metal oxide NPs may also have various types, features, and applications.Nowadays, metallic materials are usually divided into two categories (ferrous and non-ferrous metals) according to their colour and properties.7 Ferrous metals include iron, manganese, chromium, and their alloys. They are called ferrous metals because the surface of steel is often covered by a layer of black Fe3O4 film, while manganese and chromium are often used to make steel alloys with iron. Therefore, manganese and chromium, together with iron, are collectively referred to as ferrous metals. These three metals constitute the primary raw materials for steel smelting, accounting for about 95% of the total metal production globally and play an essential role in the industrial and medical sectors.7 More than 60 types of metals, besides ferrous metals, are together called non-ferrous metals. Most of them are silver or white, except gold (yellow) and copper (red).8 Nonferrous metals can be divided into four categories, as follows: (I) heavy metals (nonferrous metals with ρ > 4.5 g cm−3): this category includes most transition elements in the periodic table, such as copper (Cu), zinc (Zn), nickel (Ni), cobalt (Co), tungsten (W), molybdenum (Mo), cadmium (Cd), and mercury (Hg), as well as antimony (Sb), bismuth (Bi), lead (Pb), and tin (Sn). They are difficult to biodegrade and are instead enriched via bio-amplification in the food chain, eventually entering the human body.9 Heavy metals interact strongly with human proteins and enzymes, rendering them inactive. They can also accumulate in some organs, causing chronic poisoning and damage to tissues and organs.10 (II) Light metals (metals with ρ < 4.5 g cm−3): this category includes metals such as lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), aluminium (Al), magnesium (Mg), and calcium (Ca). Light metals have many excellent physical and chemical properties and are widely used in industrial fields such as manufacturing and metallurgy.11 (III) Noble metals: noble metals are metals with stable physical and chemical properties. They typically show low reserves in the Earth's crust, have an elegant appearance, and are expensive. They include rhodium (Rh), ruthenium (Ru), palladium (Pd), silver (Ag), osmium (Os), iridium (Ir), platinum (Pt), and gold (Au).12 Noble metals have unique properties and are physicochemically stable, with high oxidation and corrosion resistance, excellent processing characteristics, and little effects on human tissues.13 Therefore, they are widely used in aerospace engineering, industry, and especially the medical field. (IV) Scarce metals: these metals are less abundant in the Earth's crust and are scattered or difficult to extract from raw materials.14,15 They can be further subdivided into six categories according to their physicochemical properties and production methods, as follows: (i) rare light metals, such as beryllium (Be), lithium (Li), rubidium (Rb), and caesium (Cs), which have a low specific gravity and strong chemical activity. (ii) Rare precious metals, such as platinum (Pt), iridium (Ir), and osmium (Os). (iii) Rare scattered metals, such as gallium (Ga), germanium (Ge), indium (In), and thallium (Tl), which are typically present in minerals of other elements. (iv) Rare earth metals, including scandium (Sc), yttrium (Y), lanthanum (La), cerium (Ce), and neodymium (Nd), which have very similar chemical properties and co-exist in minerals. (v) Refractory rare metals, including titanium (Ti), zirconium (Zr), tantalum (Ta), vanadium (V), and niobium (Nb), which have a high melting point, together with their compounds derived from carbon, nitrogen, silicon, and boron. (vi) Radioactive rare metals, such as polonium (Po), radium (Ra), actinium (Ac), uranium (U), and plutonium (Pu). This system of classification is not very strict, and some scarce metals are often included in multiple categories.15,16 For example, rhenium can be included in rare scattered metals or refractory rare metals (Fig. 1).
In addition to being widely used in industry, the aforementioned metals also play an essential role in biomedicine. For example, metal and metal oxide NPs are used as carriers for drug delivery, and they can exert anti-tumour and antibacterial effects.17,18 Noble metal nanomaterials (NMNs) also show application prospects and great potential in energy, catalysis, biosensors, and medicine. Most NMNs possess excellent biocompatibility and exert low toxicity. Au and Ag NPs are the most widely studied due to their ease of preparation and high safety. Furthermore, gold NPs have attracted widespread attention due to their relative non-toxicity.19,20 Gold NPs have been used clinically to treat rheumatoid arthritis since the 1920s, when they were called ‘colloidal gold’.21 Gold NPs are also widely used in electron microscopy probes and biomolecule delivery carriers, and as biosensors and imaging labels.20,22,23 Similar to gold NPs, silver NPs have excellent antibacterial properties and are widely used in wound dressings, catheters, cosmetics, and clothing. In addition, silver NPs are also a potential anti-tumour angiogenesis drug, and they have prospects for application in the treatment of stroke, pulmonary oedema, myocardial infarction, rheumatoid arthritis, and other diseases.24 NMNs have some remarkable unique properties compared to other metal NPs. For instance, NMNs show a plasma resonance spectral band, which is not available in other metal materials.25 Nowadays, NMNs are widely used in biological imaging,26 drug release, and biosensor technology due to their freely adjustable absorption and scattering spectra.27,28 In addition, recent studies have shown that NMNs can reprogram the tumour microenvironment, thereby inhibiting tumour growth.29
In addition to NMNs, other metal and metal oxide NPs are also increasingly being used in biomedicine. In recent years, magnetic nanomaterials based on ferriferous oxide have attracted wide attention in the field of medicine. When reduced to a size on the nanometre scale, superparamagnetic iron oxide NPs can only be influenced by an external magnetic field, enabling them to form stable colloids in physical-physiological media. Their superparamagnetism and other intrinsic properties, such as low toxicity, colloidal stability, biodegradability, and traceability, make them ideal for biomedical applications in vivo and in vitro.30,31 The characteristics, applications, and toxicity of heavy metal and metal oxide NPs (such as copper, copper oxide, and zinc oxide NPs) have been the focus of extensive research. Many studies have reported the applications of NPs in medicine. For example, copper is a relatively cheap metal and commonly used. Its NPs have low preparation costs and show excellent performance; thus, they are used in various industries, especially the pharmaceutical industry. In addition, copper is a trace element, harmless to many living cells, and can participate in many metabolic reactions. In contrast, Cu NPs show significant antibacterial and bactericidal activity when cell membranes, nucleic acids, and proteins are damaged.32 ZnO is relatively inexpensive, has good biocompatibility and low toxicity, and has shown good application prospects in many aspects of biomedical engineering. Several studies have confirmed that zinc oxide and its NPs are antibacterial. Compared with ordinary ZnO, ZnO NPs have a smaller particle size and a significant micro-quantum effect, exerting substantially improved antibacterial properties and application prospects.32 In recent years, there has been an increase in research on rare metal and metal oxide NPs and their applications. For instance, CeO3 and TiO2 NPs have antioxidant effects. They are characterised by a small size, controllable and flexible modification, relatively low toxicity, and easy preparation. Thus, they have promising application prospects in medicines, cosmetics, and food additives.33Table 2 presents a summary of the properties and biomedical applications of some common metal and metal oxide NPs.
| Type | Cell subtypes | Main ways of energy metabolism | Ref. | |||
|---|---|---|---|---|---|---|
| Glycolysis | OXPHOS | FAO | Glutaminolysis | |||
| ++: major metabolic pathway; +: minor metabolic pathway; −: neither major nor minor. | ||||||
| T cells | Naive T cells | − | + | ++ | − | 210 and 211 |
| TH1 cells | ++ | − | − | + | 210 | |
| TH2 cells | ++ | − | − | + | 210 | |
| TH17 cells | ++ | − | − | + | 210 | |
| Treg cells | − | − | ++ | − | 212 | |
| Memory T cells | − | − | ++ | − | 212 | |
| Cytotoxic T cells | ++ | − | − | + | 210 | |
| B cells | Pro-B cells | ++ | − | − | + | 213 and 214 |
| Immature B cells | ++ | − | − | + | 213 and 215 | |
| Mature B cells | ++ | − | − | + | 213 and 215 | |
| Innate immune cells | Dendritic cells (resting) | − | ++ | − | − | 216 and 217 |
| Dendritic cells (active) | ++ | − | − | − | 217 and 218 | |
| Macrophages (M1) | ++ | − | − | − | 219 | |
| Macrophages (M2) | − | ++ | ++ | − | 216 and 220 | |
| Neutrophils | ++ | − | − | − | 221 and 222 | |
| NK cells | ++ | − | − | + | 223–225 | |
| Classification of metal materials | Type of NPs | Properties | Applications | Ref. |
|---|---|---|---|---|
| Ferrous metal | Fe2O3 NPs | Magnetic, traceability, imaging | Cell labeling, tumor therapy, MRI | 226–228 |
| Fe3O4 NPs | Magnetic, traceability, near-infrared plasma absorption, thermal ablation characteristics | Biosensors, hyperthermia, PA imaging of tumors, PTT, gene transfer, protein separation | 22, 229 and 230 | |
| SPIONPs | Superparamagnetic, traceability, low toxicity, colloidal stability, biodegradability | Tumor cell markers, targeted tumor cells, MRI, drug delivery | 231–233 | |
| Mn3O4/Mn2O3/MnO2 NPs | Antioxidant activity | ROS scavengers | 234–236 | |
| Cr2O3 NPs | Antibacterial activity | Antibacterial agents | 237 | |
| CoCr NPs | Low wear and a low incidence of osteolysis | MoM arthroplasties | 238 and 239 | |
| Heavy metal | Cu NPs | DNA degradation potential, anticancer and antibacterial activity | Tumor therapy, antimicrobial agents | 240 and 241 |
| Bi NPs | High stability, strong diamagnetism, high near-infrared absorption and photothermal conversion efficiency | Drug carriers, cancer combination therapy, photothermal and radiation therapy, bioimaging, tissue engineering | 242 and 243 | |
| ZnO NPs | Low toxicity, antibacterial activity | Antibacterial agents | 32 and 244 | |
| CuO NPs | Antibacterial, antiviral, antioxidant, anticancer, high temperature superconductivity | Antibacterial agents, antiviral drugs, dentin binding agents, tumor therapy, imaging agents, drug delivery agents | 245–247 | |
| Ni2O3/Co3O4/CoO NPs | Antibacterial activity | Antibacterial agents | 237 | |
| Light metal | Al2O3 NPs | Good biocompatibility, high strength, antibiosis | Antibacterial agents, self-healing | 248 and 249 |
| MgO NPs | Antibacterial activity | Antibacterial agents | 248 and 250 | |
| Noble metal | Au NPs | Large absorption of near-infrared light, antibacterial, antiviral, anti-angiogenesis, SERS, osteoinductive | Photothermal therapy, anti-angiogenic agents, immunoassays, cancer therapy, inhibition of HIV-1, biomedical imaging, bacterial screening, osteoinductive agent for implant dentistry | 251–253 |
| Ag NPs | Antibacterial, anti-angiogenesis, anti-fungal, antiprotozoal, promoting reparative regeneration, sturdy and durable | Antibacterial agents, bone regeneration, nerve regeneration, tumor diagnosis and treatment, biosensors, dental resin filler composites | 254–257 | |
| Pt NPs | Antioxidant, antibacterial, strong affinity with dopamine, electrocatalysis | ROS scavengers, bacteriostatic agents, dopamine sensors, targeting tumor cells | 258–261 | |
| Pd NPs | Catalytic performance, photothermal ablation | PTT agents, cancer treatment | 262 and 263 | |
| Ru NPs | Antibacterial and antioxidant, osteogenesis | Bacteriostasis, ROS scavengers, regulating the behavior of stem cells | 264–266 | |
| Rh NPs | Photothermal | Cancer phototherapy | 267 | |
| Ir NPs | Photosensitive, hydrophobicity, charge transfer | Enhanced photodynamic performance, drug delivery, bioimaging | 268–270 | |
| AgO NPs | Antiviral | Fighting the drug-resistant types of viruses | 271 | |
| Scarce metal | CeO2 NPs | Anti-inflammatory, antioxidant, antibacterial, osteogenic, pro-angiogenic | Antibacterial agents, ROS scavengers, anti-inflammatory therapy, bone regeneration, vascular regeneration | 244 and 272–274 |
| TiO2 NPs | Antioxidant, stability, antiangiogenic, photocatalytic activity | ROS/RNS scavenging, PDT, SDT, photo-controlled drug release and targeted therapy | 33 and 275–277 | |
| Ta NPs | High corrosion resistance, anti-inflammatory, anti-apoptotic, osteogenic | Bone regeneration, in vivo treatment of transplanted vascular lesions | 65, 278 and 279 |
3. Immunotoxicity mechanisms of metal and metal oxide NPs
Metal and metal oxide NPs can enter the body through various pathways (e.g., inhalation, gastrointestinal absorption, and biomedical application) and get absorbed by the spleen and bone marrow or distributed to other tissues, organs, and cells after entering the blood system.12 Subsequently, these NPs continue interacting with the cells in the body, which may result in both positive and negative effects. Bulk metal and metal oxide NPs are recognised as foreign antigens, triggering an immune response. The immunotoxicity of Au, Ag, TiO2, and Fe2O3 NPs has been reported previously.34–38 The literature indicates that metal and metal oxide NPs can react with different immune cells, including macrophages, dendritic cells (DCs), natural killer (NK) cells, and B and T lymphocytes. During this interaction, the NPs are engulfed and processed by immune cells, thereby affecting their metabolism, function, and fate. Oxidative stress and inflammation have been studied extensively as immunotoxic mechanisms induced by metal and metal oxide NPs. Oxidative stress refers to the imbalance between oxidation and antioxidation in the body, where under the conditions of oxidative stress, there is an excess of reactive oxygen species (ROS), and biomolecules tend to be oxidized. Inflammation is the defensive response of the body against external agents. However, if not regulated, it can also have harmful effects. The processes of oxidative stress and inflammation are fundamentally related.39 Therefore, the response of immune cells to NPs is bimodal. To understand the immunotoxicity mechanisms of metal and metal oxide NPs, it is essential to examine the anti-inflammatory and antioxidant responses that mediate the response of immune cells to NPs and their eventual impact on the human body. The toxic effects of some metal and metal oxide NPs on different types of immune cells have been reported. For example, a significant reduction in the number of NK cells was observed in mouse models after prolonged exposure to TiO2 NPs.40 Moreover, TiO2 NPs were found to up-regulate the expression of MHC-II, CD80, and CD86 in DCs.41 Overall, these results indicate that metal and metal oxide NPs can affect the function of immune cells through different mechanisms, producing immunotoxic effects. In the following sections, we summarise the modes of metal and metal oxide NP immunotoxicity reported thus far, in which the changes in NP immunometabolism may also affect immunotoxicity (Tables 3 and 4).| Classification of metal materials | NP types | NP properties | Models | Mechanisms | Results | Ref. |
|---|---|---|---|---|---|---|
| Ferrous metal | Fe2O3 | 30–35 nm | Lymphocytes of healthy Wistar rats | Induced concentration-dependent oxidative stress and increased ROS, lipid peroxidation levels, antioxidant enzymes and GSH consumption in lymphocytes (male). Imbalance of lipid peroxidation and antioxidation in all vital organs (female). | Morphological changes of lymphocytes and induction of ROS-mediated cytotoxicity. | 280 |
| CoCr | 50–150 nm | Monocytes (U937 cells) | Increased secretion of TNF-α (resting cells); increased IFN-γ production (activated cells). | Contribute to in vivo osteolysis process; protection of cells against tissue injury. | 281 | |
| 30–35 nm | RAW 246.7 | Reduce cell viability, induce DNA damage, chromosome aberration, metal hypersensitivity increased. | Soft-tissue reactions (local) and arthroprosthetic cobaltism (systemic). | 238 and 282 | ||
| Heavy metal | NiO | 5–100 nm | Human peripheral blood lymphocytes | ROS production and lipid peroxidation. | Induce oxidative stress and inflammatory response. | 283 |
| ZnO | 20 or 100 nm; positive or negative charge | RAW 246.7 | The positively charged NPs exerted higher cytotoxicity. | Lead to immunotoxicity in vitro. | 42 | |
| Co3O4 | ≤50 nm | Human lymphocytes | Oxidative stress. | Decreased cell viability and increased cell membrane damage (dose-dependent). | 142 | |
| Noble metal | Au | 12, 35, 60 nm; PEG and OVA-coated | RAW 246.7 | The OVA-coated GNPs induce higher secretion of TNF-α, IL-6, and IL-1β. | Smaller and the OVA-coated GNPs induced stronger inflammatory responses. | 284 |
| Ag | <30 nm | THP-1 cells | Downregulation of CD11b and response to LPS stimulation, blocking the degradation of p62, inducing lysosomal damage. | Prevent THP-1 cells from differentiating into macrophages. | 116 | |
| 100 nm; AOT/PVP/PLL/BSA-coated | hPBMC | Oxidative stress, mitochondrial membrane damage, DNA damage. | Apoptosis and cell death (dose and time-dependent); genetic toxicity potential. | 285 | ||
| Scarce metal | TiO2 | 20–80 nm | Murine dendritic cells | Upregulate MHC-II, CD80 and CD86, activate inflammasome, enhance ROS production. | Strong influence on the activation state of DCs. | 41 |
| 10–30 nm | HUVECs | Induce intracellular ROS production, cell membrane oxidative damage, IKKα/β and Akt phosphorylation and p38 dephosphorylation. | Oxidative stress and apoptosis. | 88 | ||
| 17, 117 nm | THP-1 cells | Glutathione depletion, increased IL-8 and IL-1β, DNA damage and cytotoxicity. | Large agglomerates of 17 nm TiO2 induced stronger responses than small agglomerates, while no effect of agglomeration was observed with 117 nm TiO2. | 286 |
| Classification of metal materials | NP types | NP properties | Models | Mechanisms | Results | Ref. |
|---|---|---|---|---|---|---|
| Ferrous metal | Fe2O3 | Needle-like shape | 5-Week-old male ICR mice | Increased secretion of chemokines; enhanced expression of CD80, CD86 and MHC II (BAL). | Enhance the function of pulmonary antigen presenting cells by inducing Th1 polarized immune response. | 46 |
| SPIO | 15–20 nm; 300 nm (PLGA-coated) | Female NIH mice | Extensive damage to lysosomes, accumulation of LC3-positive autophagosomes, mitochondrial damage, ER and Golgi stress, and PLGA-coated Fe3O4 NPs reduced the damage to these organelles. | Autophagosomes accumulated in the kidney and spleen (detection of endogenous LC3 protein distribution). | 287 | |
| Resovist®, 28 mg Fe per mL | Male BALB/c mice | Inhibition of inflammatory cytokines (IFN-γ, IL-4) and antigen-mediated antibody responses. | Impaired antigen-specific immune responses. | 157 | ||
| 45 ± 9.8 nm, 89 ± 0.4 nm, 67 ± 4.6 nm; DEX-coated and PEG-coated | Female Wistar rats | Affect anti-oxidant and tissue nitrite levels. | Mast cell infiltration in liver, lung and heart. | 288 | ||
| CrNano | 40–70 nm | Male Sprague-Dawley rats | Increase the serum level of IgG; enhance lymphoid tissue proliferation response of peritoneal macrophages, anti-SRBC PFC response and phagocytic activity. | Affect hormone and immune responses in heat-stressed rats. | 289 | |
| Heavy metal | NiO | 20 nm | Male Wistar rats | NF-κB activation and Th1/Th2 imbalance. | Enhance the nitrative stress and inflammatory response in lung tissue. | 290 |
| 5–100 nm | Rodents | ROS production and lipid peroxidation. | Induce oxidative stress and inflammatory response. | 283 | ||
| Cu | 45–115 nm | Male Sprague-Dawley rats | Induce oxidative stress and overexpression of pro-inflammatory/anti-inflammatory cytokines. | Repress the immune function of the spleen. | 291 | |
| 32.7 ± 10.45 nm | Male Sprague-Dawley rats | Activate TGF-β1/Smad-dependent and -independent pathways (MAPK and Akt/FoxO3). | Hepatic damage and markedly increased oxidative stress in liver tissues. | 292 | ||
| 40 nm (5, 10, 15 mg kg−1) | Male BALB/c mice | Oxidative stress, chromosome aberration, DNA degradation. | Induce significant genotoxicity at the highest concentration. | 293 | ||
| ZnO | 20 or 100 nm; positive or negative charge | C57BL/6 mice | Inhibition of NK cell activity and serum levels of pro/anti-inflammatory cytokines and T helper-1 cytokines. | Lead to immunotoxicity in vivo; immunosuppression. | 42 | |
| <40 nm | Male Wistar albino rats | Oxidative/inflammatory pathway. | Induce obvious immunotoxicity in the thymus and spleen. | 48 | ||
| CdO | 9.82 nm | Female ICR mice | The percentage of CD3e + CD8a + cells in the thymus increased, and the production of spleen cells, inflammatory cytokines and chemokines increased. | Stimulation of immune/inflammatory response, oxidative stress in the intestine. | 294 | |
| Noble metal | Ag | 20 nm, 100 nm | Wistar rats | Affect multiple immune parameters. | Adverse effects on the immune system. | 295 |
| Scarce metal | TiO2 | 21 nm | Sprague-Dawley rats | Change the expression levels of IFN-γ, IL-4, T-bet and GATA-3; Th1/Th2 cytokine imbalance. | Increase accumulation of pulmonary macrophages, lung injury. | 296 |
| 5–6 nm | Female ICR mice | Alteration of inflammatory and apoptotic cytokines expression. | Lymphocyte subsets and immune capacity decreased, spleen damage. | 40 | ||
| Significantly increase the levels of various inflammatory factors and chemokines, while decreasing NKG2D, NKp46 and 2B4. | Significantly increase the spleen and thymus indices, spleen damage. | 297 | ||||
| Activation of NF-κB-mediated MAPKs pathway. | Exert toxic effects on lymphoid organs and T cells and innate immune cell homeostasis. | 76 | ||||
| 10, 50, 100 nm | Female C57BL/6J mice | Alteration of T lymphocyte proliferation and phenotype. | Cause low-grade intestinal inflammation and aggravate immunological response to external stimulus. | 298 | ||
| 17, 117 nm | C57BL/6JRj mice | Glutathione depletion, increased IL-8 and IL-1β, DNA damage and cytotoxicity. | Large agglomerates of 117 nm TiO2 induced higher pulmonary responses and blood DNA damage compared to small agglomerates. | 286 | ||
| 22.75 ± 7.04 nm | Female Kunming mice | Th1/Th2 imbalance. | Induced ileal physical barrier dysfunction (dose-dependent). | 299 | ||
| Light metal | AlO | Aspect ratios (6.2 ± 0.6, 2.1 ± 0.4) | 6-Week-old male ICR mice | Alter the levels of redox response-related elements. | May influence immune functions in an exposed host. | 64 |
| Al2O3 | 13, 50 nm | 3-Month-old male ICR mice | The levels of SOD and GSH decreased, the malondialdehyde increased. | Immune organs damage and immune cells dysfunction, leading to abnormal immune-related cytokine expression. | 43 | |
| 20 nm | Male Sprague-Dawley rats | DNA damage. | Induce genetic toxicity of bone marrow. | 300 |
3.1 Inflammatory responses
Metal and metal oxide NPs can trigger the release of many inflammatory factors. It has been reported that ZnO NPs of different sizes and charges can cause immunotoxicity by inducing inflammation42 and cytokines and chemokines determine the severity of the inflammatory response. Therefore, understanding the timing and mechanisms of the inflammatory responses mediated by metal and metal oxide NPs is vital for developing safe NPs.Overall, the current evidence shows that metal and metal oxide NPs can alter anti-inflammatory and pro-inflammatory pathways in vivo, affect different signalling pathways in the immune system, and trigger inflammatory responses (Fig. 2). However, due to the differences in the types of immune cells and the physical and chemical properties of NPs as well as their concentration, dose, route of administration, and timing of use, comparisons across studies are complex, and thus more scientific and systematic analyses are required.
3.2 Oxidative stress
Oxidative stress can induce the production of a large number of oxidative intermediates, resulting in an oxidation–antioxidation imbalance in vivo, which eventually leads to an inflammatory response66 (Fig. 2). Metal and metal oxide NPs can produce ROS through different mechanisms, and excessive ROS can cause cellular oxidative stress responses such as lipid peroxidation, DNA damage, and abnormal signal transduction. A previous study reported that ROS induced by metal and metal oxide NPs activate the Fenton or Haber–Weiss reaction, thereby aggravating oxidative stress damage,67 even in immune cells (Fig. 5). Hence, regardless of their subcellular source, the excessive ROS induced by metal and metal oxide NPs have toxic effects on healthy cells. Oxidative stress is one of the primary mechanisms through which these NPs cause toxicity in immune cells.It is well-known that inflammation and oxidative stress can interact, with inflammation increasing the production of ROS and ROS aggravating inflammation. Senapati V. A. et al. exposed human THP-1 cells to 30 nm ZnO NPs to investigate the immunotoxic potential of the NPs.74 The NPs induced oxidative and nitrosative stress in a dose-dependent manner, down-regulated the antioxidant glutathione (GSH), and increased the TNF-α and IL-1β levels by activating the NF-κB and MAPK signalling pathways to promote inflammation.74 Current evidence suggests that TiO2 NPs can induce oxidative stress through p38. A study exploring the in vitro immunotoxicity of TiO2 NPs (20 nm, negatively charged) against RAW 264.7 mouse macrophages and the underlying molecular mechanisms showed that these NPs can induce immune cell apoptosis and toll-like receptor (TLR)-mediated signal transduction through the oxidative stress-sensitive SAPK/JNK and p38 MAPK pathways, resulting in a decrease in immune cells.75 In addition, a reduction in lymphocyte subpopulations such as CD3+, CD4+, CD8+, and NK cells was observed in female ICR mice treated with TiO2 NPs (continuous intragastric administration for 9 months), indicating the toxic effects of these NPs on mouse lymphoid organs, T cells, and innate immune cells. The findings indicated that these NPs may activate the NF-κB-mediated MAPK signalling pathways, causing immunotoxicity.76
The above-mentioned studies revealed that oxidative stress is indispensable in the immunotoxic effects induced by metal and metal oxide NPs, which cause cell dysfunction through oxidative damage and signalling pathways such as the p38, PI3K/Akt, and NF-κB pathways88 (Fig. 2). However, some rare metal and metal oxide NPs have been reported to scavenge intracellular ROS.89 For example, cerium oxide NPs reduced oxidative stress in PC12 cells by 50%, providing a substantial anti-ROS effect.90 In addition, a study by Zheng C. et al. examining the in vivo immunotoxicity of Gd2O3:Eu3+ NPs showed that the NPs produced almost no immunotoxicity in BALB/c mice.91 They found that ROS can act as a secondary messenger in signal transduction and inhibit the expression of phosphoinositide 3-kinase (PI3K) in the liver. This immunosuppression caused by PI3K inhibition helped the mice to adapt to stress, and thus tolerate Gd2O3:Eu3+ NP-induced immunotoxicity both in vitro and in vivo.91
3.3 Autophagy and apoptosis
Autophagy is the process in which cells engulf their own cytoplasmic proteins or organelles, inserting them into vesicles, which later fuse with lysosomes to form autophagic lysosomes. The components encapsulated within autophagic lysosomes are degraded to meet the metabolic needs of the cell and renew organelles.92 Apoptosis refers to the independent and orderly death of cells and is controlled by genes. Apoptosis serves to maintain the stability of the internal environment of the human body. In contrast to necrosis, apoptosis is not a passive process but an active one and is closely related to cell proliferation and senescence.93 Evidence showed that the inhibition or activation of autophagy and apoptosis by metal and metal oxide NPs also plays an essential role in their toxic effects. MAPK, death protein kinase, PI3K, AKT, mTOR, and AMP kinase are known to be the main components inducing or inhibiting autophagy in response to metal/quasi-metal NPs.94 Autophagy is associated with many cellular functions, including immunity, inflammation, and apoptosis. For example, Johnson B. et al. revealed that ZnO NPs are immunotoxic to primary and immortalised immune cells. In vivo spleen cell death was observed in mice after intranasal exposure to ZnO NPs.95 Therefore, autophagy and apoptosis can be activated or inhibited when NPs enter immune cells, producing adverse effects in cells or organisms through a range of signalling pathways (Fig. 3).Overall, the above-mentioned studies showed that autophagy may be a protective mechanism against the cytotoxicity of metal and metal oxide NPs in immune cells.106,114 However, autophagy may also trigger apoptosis or cell death, and autophagy may also be activated due to the organelle dysfunction caused by these NPs in immune cells, leading to immune system dysfunction and immunotoxicity.
3.4 Organelle damage and dysfunction
Another effect of metal and metal oxide NPs on immune cells is organelle (e.g., mitochondria, ER, and lysosomes) damage or dysfunction. After exposure to NPs, the morphology of organelles is altered due to direct NP accumulation or indirect subcellular interactions. In addition, NP-induced adaptive changes in subcellular morphology modify cell behaviour and organelle-related functions2 (Fig. 5).The above-mentioned results indicate that after exposure to metal and metal oxide NPs, immune cells can experience a range of organelle impairments through various mechanisms and signalling pathways. These may involve changes in immunometabolism, cause metabolic reprogramming, and even lead to cell death due to immunotoxicity. These processes are central to the biomedical functions and toxic reactions of NPs in vivo. However, the specific molecular mechanism is still unclear and needs further elucidation.
3.5 Changes in genetic material
Another mechanism of the immunotoxicity induced by metal and metal oxide NPs is the destruction of genetic information. This can occur via alterations in the sequence or structure of DNA and epigenetic modifications129–131 (Fig. 5). Studies have shown that the effects of NPs on genes are related to their characteristics and experimental conditions, such as composition, size, shape, surface characteristics, timing, cell type, and treatment options.132 Moreover, metal and metal oxide NPs can also induce epigenetic toxicity in immune cells.131,133,134The genotoxicity of heavy metal and metal oxide NPs has been widely reported both in vitro and in vivo. For example, ZnO NPs can cause significant genotoxicity and DNA damage in human monocytes and peripheral blood lymphocytes.74 In one study, comet assays showed a significant increase in micronuclei and DNA damage in THP-1 cells exposed to ZnO NPs (20 μg mL−1).74 Similarly, studies on the genotoxicity of ZnO NPs of different sizes (4.175, 9.058, and 19.8 nm) in human peripheral blood lymphocytes showed that ZnO NPs can cause genotoxicity at low doses (≥12.5 ppm) and induce lymphocyte death at higher concentrations (500 ppm and above). Notably, Jiang H. et al. explored the possible underlying mechanism for the effect of Co NPs on human T lymphocytes by measuring the levels of SOD, catalase, and glutathione peroxidase.141 They found that Co NPs induced primary DNA damage in a concentration-dependent manner and led to a higher degree of DNA damage than Co ions.141 DNA damage and chromosomal aberrations were also observed in human lymphocytes following exposure to Co3O4 NPs at concentrations of 100 μg mL−1, and the effects were mediated by changes in antioxidant levels.142 Similarly, the genotoxicity of TiO2 NPs is mainly mediated by the generation of oxidative stress.143 Kazimirova A. et al. tested the genotoxicity of TiO2 NPs in vitro and in vivo using a comet assay and micronucleus test.144 Increased DNA strand breaks were observed in the peripheral blood mononuclear cells (PBMCs) of female Wistar rats 1 day after exposure to TiO2 NPs (approximately 21 nm in size) induced DNA breaks in human PMBCs in a time- and dose-dependent manner without causing DNA oxidation (75 μg cm−2 after 4 h of exposure, 75 μg cm−2 after 24 h of exposure; 15 and 75 μg cm−2).144 It was also reported that alumina NPs with a concentration of up to 0.5 mM produced genotoxic effects in human peripheral blood lymphocytes by inducing oxidative DNA damage and strand breaks, which led to a concentration-dependent increase in DNA single-strand breaks but had no impact on alkali-unstable sites.145
In summary, the interaction of metal and metal oxide NPs with the immune system can cause DNA damage and genotoxicity. The specific mechanisms and degree of severity may be closely related to the oxidative stress caused by NPs as well as their concentration and physicochemical properties. When the genetic changes induced by NPs exceed the repair capacity of cells, apoptosis or necrosis may occur,146 causing toxic effects on the immune system (Fig. 4).
Thus, metal and metal oxide NPs can induce epigenetic toxicity in immune cells, leading to alterations in chromatin conformation and gene expression levels, thereby exerting toxic effects on the immune system. However, due to the influence of confounding factors such as NP concentration, particle size, surface modification, and study conditions, the relevant mechanisms are not fully understood. Thus, more rigorous and systematic studies are required to explore this further.
3.6 Immunosuppressive response
Immunosuppression refers to the inhibition of an immune response (e.g., anti-inflammatory response). The immunoregulatory mechanisms of metal and metal oxide NPs are complex, and their immunostimulatory or inhibitory effects may be related to their composition, size, surface coating, and other factors. Studies have revealed that metal and metal oxide NPs can cause immunosuppression according to their structure (Fig. 5), consistent with the immunosuppressive effects of NMNs (e.g., Au and Ag) in various immune cells.155 For example, Ag-PVP NPs (10–80 nm) induced size-dependent anti-inflammatory effects in mouse macrophages infected with live Chlamydia trachomatis, with smaller NPs producing a more pronounced down-regulation of pro-inflammatory factors such as IL-6 and TNF.156 Iron oxide NPs also show immunosuppressive effects on immune cells. For example, OVA-specific IgG (1) and IgG (2a) are significantly reduced in BALB/c mice after the intravenous injection of a single dose of iron oxide NPs (10–60 mg Fe per kg) over 7 days.157 In addition, IONPs attenuated Th1 and Th2 cell-mediated immunity in OVA-sensitized mice, and inhibitory effects on IL-17, IL-6, ROR-γt, and Th17 immune responses were observed in OVA-sensitized mice after exposure to Resovist® (containing iron oxide NPs, 28 mg Fe per mL; single intravenous injection).158 Hence, systemic exposure to a single dose of iron oxide NPs inhibited antigen-specific antibody production and T cell function, thereby weakening immune responses. Notably, CeO2 NPs showed a scavenging effect against ROS. These NPs were found to scavenge free radicals and ROS in J774A.1 mouse macrophages and inhibit the production of inflammatory mediators, thereby exerting antioxidant and anti-inflammatory effects in vitro.1593.7 Metal homeostasis disruption
The human body naturally contains different metallic elements. Na, K, Mg, Ca, Fe, Mn, Co, Cu, Zn, and Mo are essential elements for life processes. These metals can significantly affect a variety of cellular functions, including immune function.160,161 Metal and metal oxide NPs can dissolve or degrade into metallic elements or ions after entering the body, destroying the metal balance in vivo. Iron metabolism is tightly controlled in the body. Iron regulates macrophage polarisation, neutrophil recruitment, and NK cell activity in innate immunity. In contrast, in adaptive immunity, iron affects the activation and differentiation of Th1, Th2, and Th17 cells as well as CTLs, in addition to antibody responses in B cells.162 Thus, disturbances to iron metabolism can disrupt metal homeostasis and promote immune responses (Fig. 5). In vitro and in vivo studies have demonstrated that Zn, Cu, Fe2O3, and Ag NPs can disrupt metal homeostasis,163 with Feraheme@ affecting iron homeostasis in human primary T cells.118 However, whether they can affect intracellular transport and other functions after accumulation in immune cells warrants further investigation. In addition, the dissociation of ZnO NPs can disrupt zinc homeostasis in primary macrophages.164 Similarly, studies by Cuillel M. et al. showed that the disruption of Cu and Zn homeostasis, including intracellular Cu overload and interference with Cu–Zn exchange on metallothionein, occurred in hepatocytes treated with subtoxic doses of CuO NPs.165 The metallothionein family could activate related transcription factors in the presence of excess metal, thereby regulating metal homeostasis.166 In addition, the expression of Met-RNA was found to be higher in cells treated with Zn, Ag, and CuO NPs. However, no significant up-regulation of metal homeostasis-related genes was observed in some hepatocytes treated with Zn, Cu, or AgO NPs.163Although these studies have proven that metal and metal oxide NPs can cause specific effects on metal homeostasis in vivo, the overall literature remains limited. At present, the specific mechanisms by which free ions released by NPs act on cells are still unclear. Therefore, the destruction of metal homeostasis as a mechanism of immunotoxicity requires validation in future studies.
4. Metabolism and fate of metal and metal oxide NPs in vivo
Humans are often exposed to metal and metal oxide NPs through ingestion, inhalation, and skin contact, and the emergence of therapeutic drugs based on these NPs has increased the interest in their fate after administration.167,168 NPs are stable under colloidal, chemical, and biological conditions.1 However, their stability can be lost under physiological conditions (e.g., in blood, tissues, and cells) or during storage.169,170 During this process, NPs may clump or gather (e.g., protein corona) or disintegrate and corrode (e.g., release metal from metal and metal oxide NPs)171 (Fig. 6). The degradation, dissolution, and erosion of metal and metal oxide NPs can be divided into core erosion, surface erosion, and bulk erosion, and these processes are referred to as biodegradation, biodissolution, and bioerosion, respectively, when they occur in response to biological agents or physiological conditions1 (Fig. 6). These physiological conditions can represent a simple simulation of the biological environment, such as lysosomal pH, or biological macromolecules such as enzymes. In addition, changes in environmental pH may alter the degradation and dissolution of metal and metal oxide NPs based on their physicochemical properties. Thus, during the interaction of NPs with tissues or cells in vivo, cells may encounter the biodegradation products of NPs, which may eventually cause a range of molecular alterations. After digestion in cells or tissues, NP fragments may be recognised as foreign antigens in the host, triggering different immune responses, and eventually leading to different outcome pathways.4.1 Immune recognition, metabolism, and clearance of NPs
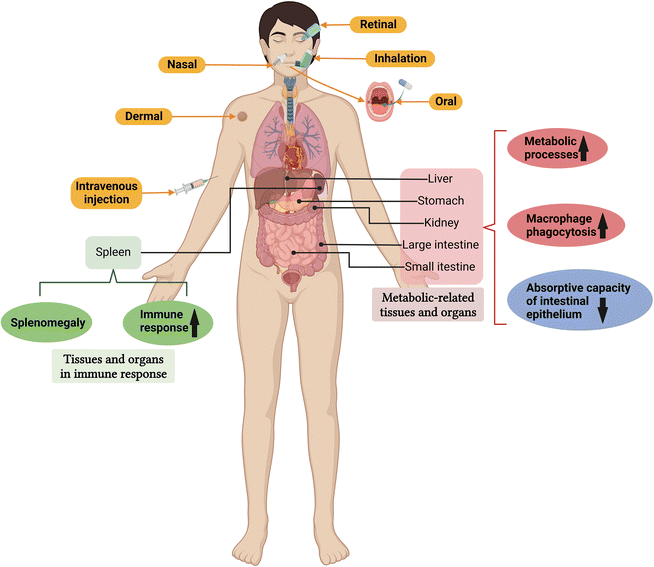
The in vivo recognition of metal and metal oxide NPs can also have an important effect on their metabolism and clearance. In the body, most metal and metal oxide NPs are recognised by immune cells as foreign antigens, triggering immune responses. Although some proteins from the biological microenvironment may get adsorbed onto the surface of these particles and cause poor immune cell recognition, most metal NPs cannot escape immune recognition.172 Biodistribution studies showed that the biodegradation and removal of IONPs within 2 weeks of distribution in the liver and spleen depended mainly on their size and surface coating.173 Metal ions released in vivo from metal and metal oxide NPs (e.g., Ag+, Au+, Cd+, Zn2+, and Fe2+) may be toxic even at low concentrations, participate in different cellular pathways, or induce changes in ROS and intracellular metal homeostasis.170 Some ultra-small metal and metal oxide NPs can be transported across the epithelial barrier, penetrate the bloodstream, and then be swallowed by immune cells. These NPs interact with immune-related tissues and organs during accumulation in the body, resulting in far-reaching effects, which may manifest as the activation or inhibition of immune function.174,175Studies have shown that metal and metal oxide NPs accumulate in tissues and organs to varying degrees after entering the body, and are eventually metabolized into substances that cells use or excreted through the urine and faeces1 (Fig. 7). For example, the size-dependent distribution of Ag NPs (20, 80, and 110 nm) was analysed after intravenous administration in rats.176 The 20 nm particles were mainly distributed in the liver, kidney, and spleen. In contrast, the larger particles were primarily distributed in the spleen, followed by the liver and lungs.176 Renal clearance is the most effective pathway for excreting metal and metal oxide NPs. After entering the blood circulation, NPs can be excreted effectively via the kidneys in the urine. In this excretory pathway, the NPs have the least interaction with the body, which minimises their possible toxic effects.177 However, larger metal and metal oxide NPs cannot be effectively removed via the kidneys and may be excreted via bile and the gastrointestinal tract167 (Fig. 7), which is the main route for removing NPs that cannot be directly cleared by the kidneys. In general, NPs or degradation products smaller than 5.5 nm are rapidly cleared primarily through the urinary system, while that larger than 6 nm are often removed by the hepatobiliary system.178 Therefore, the liver and kidneys show higher levels of NP accumulation than other organs, and the excretion of these NPs may be size-dependent. In addition, NPs can easily enter the human digestive system and accumulate in the gastrointestinal tract (due to its direct contact with the external environment), while smaller-sized NPs are more likely to pass through the gastrointestinal tract.179
In summary, metal and metal oxide NPs can be distributed from the exposure sites (e.g., blood and intestines) to secondary organs (liver and kidney), and ultimately undergo different outcomes. The clearance period of these NPs is significant given that internalised metal and metal oxide NPs can persist in the body for a long duration, being trapped in the kidneys, liver, and reticuloendothelial system and having a significant impact on these metabolism- and immunity-related tissues and organs.
4.2 The effects of physicochemical properties and experimental methods on the immunotoxicity of NPs
Metal and metal oxide NPs are transported through the circulatory system and reach different organs and tissues after entering the body via different routes.180 Their transport mainly depends on their physical and chemical properties (size, shape, charge, surface coating, stability, crystallinity, and agglomeration state). These factors affect the function and activity of NPs, including their transfer from epithelial cells to organs, intracellular localization, action on receptors, and ROS-enhancing effects.181 For example, biodistribution and toxicity studies of gold nanoclusters (Au NCs) with different charges (5.9 mg kg−1; administered for 1, 7, 30, 60, and 90 days) showed that negative Au NCs were more likely to accumulate in the liver and spleen in male C57 mice, while positive Au NCs could damage the peripheral blood system,182 suggesting that surface charge is a decisive factor affecting the location of NP accumulation in vivo.In general, the blood, liver, spleen, and kidneys are the primary hosts for NPs. After intravenous injection, AuNPs of different sizes (10, 50, 100, and 250 nm) showed size-dependent toxicity and accumulation in rats. The larger particles were detected only in the blood, liver, and spleen, while the smallest NPs could accumulate in all organs, including the brain.183 Based on the above evidence, we speculate that the dispersion of NPs in the body is negatively correlated with their size, that is, the smaller the size of NPs, the more extensive their distribution and accumulation in vivo. It is worth noting that surface coating may be an effective strategy for altering the stability and toxicity of NPs in vivo.184 However, a study compared the adverse effects of PAA or citrate-coated gold nanospheres and PAA or PEG-coated gold nanorods on human dermal fibroblasts (HDFs). The results showed that gold nanorods altered gene expression, where in this group, IL-6 expression was 12-fold higher than that in control cells,185 suggesting that the surface chemistry of PEG is not as insignificant as commonly believed and may enhance the immunotoxicity of NPs.
The toxic effects induced by metal and metal oxide NPs in different animal models and cell lines are usually different, and NP concentrations, exposure duration, exposure modes, and temperatures also affect their toxicity. In general, the toxicity of NPs increases with an increase in their concentration and exposure duration.186,187 It is worth noting that the exposure pathway of NPs is directly related to their immunotoxic effects. For example, single or multiple intravenous injections of Ag NPs and AgNO3 with different sizes (25 μg Ag per dose of Ag NPs and 2.5 μg Ag per dose of AgNO3: 1, 4, and 10 days) led to biodistribution in the liver, lungs, and kidneys in female BALB/c mice. In this model, toxicity was caused by endothelial barrier disruption.188 After the intravenous injection of ZnO NPs, high amounts of ZnO NPs were detected in the blood of rats. However, the oral administration of these NPs (30 mg kg−1) led to obvious gastrointestinal under-adsorption.189 In addition, after the intraperitoneal injection of NPs of different sizes (micro-TiO2 and 5, 10, 60, and 90 nm anatase TiO2) and concentrations (5, 10, 50, 100, 150, and 200 mg kg−1; once a day for 14 days), mice (22 ± 3 g, half male and half female) showed Ti accumulation in the brain, spleen, lungs, and kidneys. Further, the accumulation was concentration-dependent.190 Notably, the liver was found to be severely damaged owing to mitochondrial destruction and the induction of hepatocyte apoptosis, with smaller NPs being more toxic than micro-NPs.190
The above-mentioned results demonstrate that different structural characteristics (surface charge, size, coating, etc.) and administration methods (intravenous injection, intraperitoneal injection, oral administration, etc.) of metal and metal oxide NPs affect their immunotoxic effects. These nanoparticles cause inflammatory responses and lead to chronic toxicity over time. The size-dependent toxicity and excretion of metal and metal oxide NPs have been clear, that is, smaller NPs have stronger toxic effects on the immune system and metabolic tissues because they are internalised more easily by immune cells and cross biological barriers in vivo, thereby expanding the scope of their toxicological effects.
4.3 Effects of metal and metal oxide NPs on metabolism-related tissues and organs
Metal and metal oxide NPs may affect the function and histopathology of metabolically relevant organs that interact with sub-organ cells (Fig. 8). For example, Ag NPs (3–20 nm; 5, 10, 15, and 20 mg kg−1 for 21 days) damaged epithelial microvilli and intestinal glands, and the loss of microvilli reduced the absorption capacity of the intestinal epithelium. The body weight of mice decreased significantly in all the Ag NP treatment groups.191 Intravenous administration of small-sized (10 nm) Ag NPs led to enhanced tissue distribution and significant hepatobiliary toxicity, while surface coatings (citrate and PVP) showed no related effects.192 Interestingly, a single intravenous injection of Pt NPs sized less than 1 nm had no significant toxic effect on the lungs, spleen, and heart of mice. However, tubular epithelial cell necrosis and urinary casts increased, and the mice showed a dose-dependent increase in blood urea nitrogen (an indicator of renal injury).193The liver is the main detoxification organ in the human body, and hepatic storage can reduce the systemic toxicity of NPs to some extent. These NPs tend to be digested or metabolized in the liver, and then neutralised and stored in the body to reduce toxicity.2 Therefore, the accumulation of metal and metal oxide NPs in metabolic organs can also be considered a protective mechanism. The degradation of NPs mainly depends on the phagocytic activity of Kupffer cells in the liver. One day after injection, Au NPs were found in almost all Kupffer cells. Transmission electron microscopy showed that they accumulated in the vesicular lysosomal/endosomal structures of macrophages.194 Similar results were obtained by Dragoni S. et al. in their study assessing the uptake and cytotoxicity of PVP-coated 5 nm Au NPs.195 The results indicated that although the Au NPs were rapidly distributed in the liver, they were not assimilated in hepatocytes but rather digested and accumulated in the lysosomes of macrophages through enzymatic digestion, which reduced their systemic toxicity. Furthermore, although Au NPs were rapidly internalised in the liver, they induced a reduction in lactate dehydrogenase release and MTT and glutathione levels in rat hepatocytes, with no apparent cytotoxicity. This confirmed that Au NPs have a certain degree of biocompatibility with rat hepatocytes.195 Therefore, morphological and functional alterations in metabolism-related tissues and organs can increase the body's tolerance to metal and metal oxide NPs (Table 5).
| Tissues/organs | NP types | Models | Physiological and pathological effects | Ref. |
|---|---|---|---|---|
| Lung | Au | Adult male CD-1 mice | The size and the shape greatly influence the kinetics of accumulation and excretion. Only star-like GNPs can accumulate in the lung. | 301 |
| Ag | Sprague-Dawley rats | Yellow discolouration of the lung, which is not dose-dependent. No haematological and histopathological change. | 302 | |
| Lung inflammation at day 1, disappearing by day 21. | 303 | |||
| Increased alveolar inflammation and small granulomatous lesions. | 304 | |||
| Female Wistar rats | Low dose deposition in lungs of adult healthy rats to avoid nasopharyngeal deposition. | 305 | ||
| TiO2 | C57BL/6JRj mice | Large aggregates induce higher lung response. | 286 | |
| Liver | Au | BALB/c mice | Significant genetic changes, but histological analysis showed no pathological changes, and the two sizes of NPs exhibited similar biological effects. | 306 |
| Female C57BL mice | No obvious pathological changes. | 194 | ||
| Male Wistar albino rats | Lactate dehydrogenase release and glucuronidase induction, proinflammatory effects. | 195 | ||
| Ag | Male Sprague-Dawley rats | Hepatic cytoplasmic vacuolation, no significant changes in hematology and blood biochemistry. | 307 | |
| F344 rats | Significant dose-dependent changes in alkaline phosphatase and cholesterol, mild liver damage. | 308 | ||
| Cu | Male Sprague-Dawley rats | Induce liver damage and profibrotic changes. | 292 | |
| TiO2 | SD rats | No significant adverse toxicological effects. | 309 | |
| C57BL/6JRj mice | Blood DNA damage. | 286 | ||
| Gallbladder | Ag | F344 rats | High incidence of bile duct hyperplasia with or without necrosis, fibrosis and/or pigmentation. | 308 |
| Spleen | Au | Adult female Swiss albino mice | Distorted lymphoid structure, reduced lymphoid follicles, diffuse white pulp. | 310 |
| Male Wistar rats | Significant effects on detoxification, lipid metabolism, cell cycle, defense response, and circadian rhythm-related genes. | 311 | ||
| Ag | Wistar rats | Immune cells and antibody levels in the spleen increase dramatically, spleen weight increased. | 295 | |
| Increased spleen weight, number of splenocytes and splenic cell subsets. | 312 | |||
| Cu | Male Sprague-Dawley rats | The number of macrophages in the red pulp area increased, splenic trabecular artery muscle cell degeneration, inflammatory cell infiltration; change spleen lymphocyte subsets. | 291 | |
| ZnO | Male Wistar albino rats | Degenerative changes in the spleen, decreased number of cells expressing anti-PCNA positive reaction, increased number of cells expressing anti-p53 positive reaction. | 48 | |
| Thymus | ZnO | Male Wistar albino rats | Thymic degeneration. | 48 |
| TiO2 | Female ICR mice | Thymus weight increased, lymphocyte subsets decreased; cortical starry appearance in the thymus due to macrophages, hemorrhage, severe hemolysis or congestion, steatosis and apoptosis or necrosis. | 76 | |
| Stomach | Ag | Human gastric epithelial cells | Form a complex with Helicobacter pylori to weaken Helicobacter pylori infection. | 313 |
| Intestine | Ag | Female Swiss albino mice | Reduced microvilli, intestinal epithelial absorption, weight loss. | 191 |
| Sprague-Dawley rats | Diffuse brown pigmentation, female accumulation more than male. | 314 | ||
| TiO2 | Female Kunming mice | Decreased villus height, increased crypt depth, ileal cell apoptosis. | 299 | |
| Kidney | Au | NRK cells and female BALB/c mice | Early renal fibrosis. | 315 |
| Ag | Sprague-Dawley rats | No treatment-related histopathological changes; diffuse brown pigmentation, significantly higher accumulation in female rats. | 314 | |
| Dose-dependent effects on alkaline phosphatase and cholesterol; higher accumulation in female rats. | 316 | |||
| Pt | Male BALB/c and C57BL/6 mice | Necrosis of renal tubular epithelia and urinary cast; dose-dependent increase of blood urea nitrogen (renal injury index). | 193 |
5. Discussion
Numerous studies have confirmed that the metabolic pathways associated with immune effects and the energy required to produce these effects can regulate the activation of immune responses.196,197 Usually, resting leukocytes show basal activity in all major metabolic pathways. Upon activation, they undergo metabolic reprogramming, which alters the structure and function of their mitochondria and energy consumption patterns, leading to the early use of specific metabolic pathways and metabolite fluxes.196–198 There is substantial evidence showing that immune cell polarisation is associated with metabolism, and regulating metabolism is considered an effective means to guide immune cells to a pathway that promotes infection clearance, i.e., metabolic reprogramming198 (Fig. 9 and Table 1). The mTOR pathway has been confirmed to be associated with metabolism.199 It is worth noting that with the emergence of critical metabolic nodes, various methods that rely on drugs, cytokines, lipid messengers, and microRNAs appear to be effective metabolic regulators.200 Therefore, understanding the regulatory mechanism of metabolic pathways on immune function is conducive to the development of NPs that can target immune metabolism to reshape the function of immune cells and provide a new direction for the treatment of anti-tumor function of metabolically activated immune cells.Iron metabolism is closely linked to the metabolic characteristics of different types of macrophages during differentiation and their differentiation outcomes.201 In addition, targeting iron metabolism can reprogram tumour-associated macrophages (TAMs) into M1-like macrophages, thus playing an important role in anticancer therapy.202 Therefore, in recent years, iron oxide NPs have been increasingly used to induce the metabolic reprogramming of macrophages owing to their good biocompatibility and ability to regulate macrophage activation.203 For example, spherical Au/Fe3O4 NPs could regulate the pro-inflammatory state of RAW 264.7 cells, which manifested as a significant increase in the level of pro-inflammatory factors.204 In addition, the use of IONPs as cancer therapy has shown great potential in modulating macrophages, given that they promote M1 polarisation (pro-inflammatory), thereby inhibiting tumour growth. IONPs can also serve as carriers for other immunotherapy agents and ameliorate inflammatory responses.202
Other types of metal nanoparticles may also have adverse effects on the body by regulating immunometabolism. Ag NPs are widely used due to their unique antibacterial properties. However, exposure to Ag NPs can also cause adverse effects, including inflammation, accumulation, and cell damage in various organs. It is worth noting that the study by Tiwari R. et al. showed that perinatal exposure to Ag NPs may reprogram immunometabolism and promote pancreatic β-cell death and renal damage in mice.205 This study also found that exposure to low doses of Ag NPs during pregnancy enhanced immune adaptation and could protect mouse offspring against STZ-induced diabetic nephropathy by altering immunometabolism.206 In addition, NPs have been shown to improve the activity of NK cells, enhancing their anti-tumour and anti-viral functions, by promoting the metabolic reprogramming of immune cells to effectively modulate their responses to immunotherapies.207 Therefore, by utilising the unique functional properties of NPs to promote the metabolic reprogramming of cells, the therapeutic efficacy can be enhanced and toxic effects can be attenuated. This provides an exciting therapeutic opportunity. However, the lack of standards for preclinical studies and the varying experimental conditions have created obstacles for further human trials and hindered the development of this field.202 Currently, several issues need to be addressed before the clinical transformation of NPs for immune metabolic reprogramming, including their physicochemical properties, safety and efficacy, route of administration, timing of administration, pharmacokinetics, and biodistribution. Strategies for the large-scale production of these NPs are also required.208 Overall, the use of NPs as immunomodulators to regulate immune responses requires more targeted studies.
Although current research has revealed that many key metabolites in the process of immunometabolism can affect the function of immune cells, the research in this field is still in its infancy, and thus more comprehensive exploration may be needed in the future bases on the following two aspects. Firstly, in terms of mechanism exploration, the mechanisms of action of many metabolites on other cells have been reported. Do these metabolites also play a role in immune cells and play different roles in different immune cells? In addition, as the intermediate bridge between metabolic characteristics and immune function, there are still many gaps in the understanding of molecular mechanisms. For example, fatty acid oxidation is a metabolic feature of M2 macrophage polarization, but the specific molecular mechanism of fatty acid as a metabolic substrate for fatty acid oxidation to regulate M2 polarization is not clear. Secondly, how to apply these new mechanisms to treatment is also a matter of concern. For example, the diversity of innate immune cells leads to the possibility that the same metabolic pattern may play different immune regulatory roles in different innate immune cells. Therefore, in the tumor microenvironment where multiple cells coexist, interfering with glycolysis may simultaneously affect the survival of tumor cells and the immunosuppressive function of TAM, and may also affect the anti-tumor function of DC cells and NK cells. Whether this two-way effect will affect the treatment, there is no reasonable assessment. It is worth noting that the regulatory mechanism between the metabolic characteristics of immune cells and immune responses is highly dependent on the environment in which the cells are located.209 Therefore, the metabolic characteristics and immune response regulation mechanisms should be accurately analysed in a specific environment, which can help promote the precise application of NPs as metabolic regulators of immune cells.
6. Conclusion, limitations, and prospects
We comprehensively reviewed the ability of metal and metal oxide NPs to induce inflammation, oxidative stress, DNA damage, and autophagy. After entering the body through different pathways, these NPs can activate various pathways that work independently or interact with each other to modulate the immune system. In this process, the NPs can undergo different degrees of degradation and dissolution and eventually be excreted through the metabolism-related organs of the body. However, as described in this review, there remain several unresolved issues in understanding the physicochemical properties of metal and metal oxide NPs and the effects of their degradation products and administration routes on immunotoxicity, as follows: (I) the physical and chemical stability of NPs can vary after reaching target cells or tissues. However, it is still difficult to fully track the changes in NP characteristics during this process, even though the changes can alter the immune properties of the host. (II) Our understanding of the effects of these alterations and degradation processes on immunotoxic effects is still limited, and better animal or cellular models and more accurate assays are needed to carefully examine these alterations and their effects.1 (III) The immune response induced by nanoparticles depends on the interaction between nanoparticles and immune cells. Therefore, current research also focuses on the relationship between different types of immune cells and nanoparticles in the immune system. However, due to the lack of research on immunotoxicity, we should also pay attention to the immunological properties of nanomaterials themselves, and it is particularly important to understand their complete immunological properties. Therefore, the formulation and design of metal and metal oxide NPs must be considered during their development. Many of the considerations involved have always been complex problems in this field. Future challenges will include the classification of metal and metal oxide NPs based on the results of toxicological studies. Based on studies in vitro, considering the complexity of the immune system in vivo, more experimental studies should be carried out in vivo to further clarify the immunoregulatory mechanisms of metal and metal oxide NPs, which is also lacking in current research and needs to be studied. In addition, more consideration should be given to using metal and metal oxide NPs as tools for reprogramming the metabolism of immune cells, and more mechanistic studies should be conducted to elucidate the underlying mechanisms to minimize the toxic effects of NPs themselves. This can endow NPs with superior and longer-lasting therapeutic effects.Author contributions
Conceptualization, J. B. and C. M.; writing – original draft preparation, J. B., C. M., S. L., Y. L. and P. Y.; writing – review and editing, Z. L., B. J. and S. X. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
Authors do not have any conflicts of interest to declare.Acknowledgements
J.B. and C.M. contributed equally to this work. The work was supported by the Start-up project research of Stomatological Hospital, School of Stomatology, Southern Medical University (Grant no., PY2021018).References
- R. Mohammapdour and H. Ghandehari, Mechanisms of immune response to inorganic nanoparticles and their degradation products, Adv. Drug Delivery Rev., 2022, 180, 114022 CrossRef CAS PubMed.
- Q. Huang, et al., Adaptive changes induced by noble-metal nanostructures in vitro and in vivo, Theranostics, 2020, 10(13), 5649–5670 CrossRef CAS PubMed.
- A. N. Ilinskaya and M. A. Dobrovolskaia, Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future, Toxicol. Appl. Pharmacol., 2016, 299, 70–77 CrossRef CAS PubMed.
- P. J. Murray, J. Rathmell and E. Pearce, SnapShot: Immunometabolism, Cell Metab., 2015, 22(1), 190–190.e1 CrossRef CAS PubMed.
- N. Golbamaki, et al., Genotoxicity of metal oxide nanomaterials: review of recent data and discussion of possible mechanisms, Nanoscale, 2015, 7(6), 2154–2198 RSC.
- P. Makvandi, et al., Gum polysaccharide/nanometal hybrid biocomposites in cancer diagnosis and therapy, Biotechnol. Adv., 2021, 48, 107711 CrossRef CAS PubMed.
- M. Sethurajan, E. D. van Hullebusch and Y. V. Nancharaiah, Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances, J. Environ. Manage., 2018, 211, 138–153 CrossRef CAS PubMed.
- J. Singh and S. P. Singh, Geopolymerization of solid waste of non-ferrous metallurgy - A review, J. Environ. Manage., 2019, 251, 109571 CrossRef CAS PubMed.
- Z. Jiang, et al., Heavy metals in soils around non-ferrous smelteries in China: Status, health risks and control measures, Environ. Pollut., 2021, 282, 117038 CrossRef CAS PubMed.
- Z. Fu and S. Xi, The effects of heavy metals on human metabolism, Toxicol. Mech. Methods, 2020, 30(3), 167–176 CrossRef CAS PubMed.
- S. K. Sahu, Metallurgy of Light Metals, Minerals & Mining, 2008 Search PubMed.
- M. Azharuddin, et al., A repertoire of biomedical applications of noble metal nanoparticles, Chem. Commun., 2019, 55(49), 6964–6996 RSC.
- R. R. Arvizo, et al., Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future, Chem. Soc. Rev., 2012, 41(7), 2943–2970 RSC.
- Y. V. Nancharaiah, S. V. Mohan and P. N. L. Lens, Biological and Bioelectrochemical Recovery of Critical and Scarce Metals, Trends Biotechnol., 2016, 34(2), 137–155 CrossRef CAS PubMed.
- R. U. Ayres and L. T. Peiró, Material efficiency: rare and critical metals, Philos. Trans. R. Soc., A, 2013, 371(1986), 20110563 CrossRef PubMed.
- L. T. Peiró, G. V. Méndez and R. U. Ayres, Material flow analysis of scarce metals: sources, functions, end-uses and aspects for future supply, Environ. Sci. Technol., 2013, 47(6), 2939–2947 CrossRef PubMed.
- S. Bhattacharyya, et al., Inorganic nanoparticles in cancer therapy, Pharm. Res., 2011, 28(2), 237–259 CrossRef CAS PubMed.
- C. M. Santoro, N. L. Duchsherer and D. W. Grainger, Antimicrobial efficacy and ocular cell toxicity from silver nanoparticles, Nanobiotechnology, 2007, 3(2), 55–65 CrossRef CAS PubMed.
- D. A. Giljohann and C. A. Mirkin, Drivers of biodiagnostic development, Nature, 2009, 462(7272), 461–464 CrossRef CAS PubMed.
- R. Xu, et al., Ag nanoparticles sensitize IR-induced killing of cancer cells, Cell Res., 2009, 19(8), 1031–1034 CrossRef CAS PubMed.
- Y. F. Li and C. Chen, Fate and toxicity of metallic and metal-containing nanoparticles for biomedical applications, Small, 2011, 7(21), 2965–2980 CrossRef CAS PubMed.
- M. F. Kircher, et al., A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle, Nat. Med., 2012, 18(5), 829–834 CrossRef CAS PubMed.
- C. N. Loynachan, et al., Renal clearable catalytic gold nanoclusters for in vivo disease monitoring, Nat. Nanotechnol., 2019, 14(9), 883–890 CrossRef CAS PubMed.
- S. Sheikpranbabu, et al., Silver nanoparticles inhibit VEGF-and IL-1beta-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells, J. Nanobiotechnol., 2009, 7, 8 CrossRef PubMed.
- F. Sang, et al., Recyclable colorimetric sensor of Cr(3+) and Pb(2+) ions simultaneously using a zwitterionic amino acid modified gold nanoparticles, Spectrochim. Acta, Part A, 2018, 193, 109–116 CrossRef CAS PubMed.
- I. H. El-Sayed, X. Huang and M. A. El-Sayed, Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer, Nano Lett., 2005, 5(5), 829–834 CrossRef CAS PubMed.
- A. T. Haine and T. Niidome, Gold Nanorods as Nanodevices for Bioimaging, Photothermal Therapeutics, and Drug Delivery, Chem. Pharm. Bull., 2017, 65(7), 625–628 CrossRef CAS PubMed.
- V. S. Marangoni, J. Cancino-Bernardi and V. Zucolotto, Synthesis, Physico-Chemical Properties, and Biomedical Applications of Gold Nanorods–A Review, J. Biomed. Nanotechnol., 2016, 12(6), 1136–1158 CrossRef CAS PubMed.
- S. Saha, et al., Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth, ACS Nano, 2016, 10(12), 10636–10651 CrossRef CAS PubMed.
- R. Baghban, et al., Were magnetic materials useful in cancer therapy?, Biomed. Pharmacother., 2021, 144, 112321 CrossRef CAS PubMed.
- H. Wei, et al., Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications, Int. J. Nanomed., 2021, 16, 6097–6113 CrossRef PubMed.
- S. Hemmati, et al., Application of copper nanoparticles containing natural compounds in the treatment of bacterial and fungal diseases, Appl. Organomet. Chem., 2020, 34(4) DOI:10.1002/aoc.5465.
- X. Ge, Z. Cao and L. Chu, The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles, Antioxidants, 2022, 11(4), 791 CrossRef CAS PubMed.
- L. A. Dykman and N. G. Khlebtsov, Immunological properties of gold nanoparticles, Chem. Sci., 2017, 8(3), 1719–1735 RSC.
- V. Galbiati, et al., In vitro assessment of silver nanoparticles immunotoxicity, Food Chem. Toxicol., 2018, 112, 363–374 CrossRef CAS PubMed.
- N. Ninan, N. Goswami and K. Vasilev, The Impact of Engineered Silver Nanomaterials on the Immune System, Nanomaterials, 2020, 10(5), 967 CrossRef CAS PubMed.
- C. M. Lappas, The immunomodulatory effects of titanium dioxide and silver nanoparticles, Food Chem. Toxicol., 2015, 85, 78–83 CrossRef CAS PubMed.
- A. Shah and M. A. Dobrovolskaia, Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations, Nanomedicine, 2018, 14(3), 977–990 CrossRef CAS PubMed.
- J. K. Tee, et al., Oxidative stress by inorganic nanoparticles, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8(3), 414–438 CAS.
- X. Sang, et al., The chronic spleen injury of mice following long-term exposure to titanium dioxide nanoparticles, J. Biomed. Mater. Res., Part A, 2012, 100(4), 894–902 CrossRef PubMed.
- M. Winter, et al., Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells, Nanotoxicology, 2011, 5(3), 326–340 CrossRef CAS PubMed.
- C. S. Kim, et al., Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge, Int. J. Nanomed., 2014, 9(Suppl 2), 195–205 CAS.
- H. Li, et al., Toxicity of alumina nanoparticles in the immune system of mice, Nanomedicine, 2020, 15(9), 927–946 CrossRef CAS PubMed.
- J. Małaczewska, The splenocyte proliferative response and cytokine secretion in mice after oral administration of commercial gold nanocolloid, Pol. J. Vet. Sci., 2015, 18(1), 181–189 Search PubMed.
- M. Zhu, et al., Nanoparticle-induced exosomes target antigen-presenting cells to initiate Th1-type immune activation, Small, 2012, 8(18), 2841–2848 CrossRef CAS PubMed.
- E. J. Park, et al., Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells, Environ. Res., 2015, 143(Pt A), 138–147 CrossRef CAS PubMed.
- C. C. Shen, et al., A role of cellular glutathione in the differential effects of iron oxide nanoparticles on antigen-specific T cell cytokine expression, Int. J. Nanomed., 2011, 6, 2791–2798 CAS.
- M. A. Abass, et al., Effect of orally administered zinc oxide nanoparticles on albino rat thymus and spleen, IUBMB Life, 2017, 69(7), 528–539 CrossRef CAS PubMed.
- D. M. Gonçalves, S. Chiasson and D. Girard, Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles, Toxicol. in Vitro, 2010, 24(3), 1002–1008 CrossRef PubMed.
- C. Parnsamut and S. Brimson, Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-α production via MAPK pathway in leukemic cell lines, Genet. Mol. Res., 2015, 14(2), 3650–3668 CrossRef CAS PubMed.
- G. Côté-Maurais and J. Bernier, Silver and fullerene nanoparticles’ effect on interleukin-2-dependent proliferation of CD4 (+) T cells, Toxicol. in Vitro, 2014, 28(8), 1474–1481 CrossRef PubMed.
- K. L. Owen, N. K. Brockwell and B. S. Parker, JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression, Cancers, 2019, 11(12), 2002 CrossRef CAS PubMed.
- S. Banerjee, et al., JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects, Drugs, 2017, 77(5), 521–546 CrossRef CAS PubMed.
- L. Xu, et al., Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel, J. Nanobiotechnol., 2012, 10, 16 CrossRef CAS PubMed.
- D. J. You, et al., Sex differences in the acute and subchronic lung inflammatory responses of mice to nickel nanoparticles, Nanotoxicology, 2020, 14(8), 1058–1081 CrossRef CAS PubMed.
- X. Li, et al., Suppression of PTPN6 exacerbates aluminum oxide nanoparticle-induced COPD-like lesions in mice through activation of STAT pathway, Part. Fibre Toxicol., 2017, 14(1), 53 CrossRef PubMed.
- F. Zeng, et al., A drug-free nanozyme for mitigating oxidative stress and inflammatory bowel disease, J. Nanobiotechnol., 2022, 20(1), 107 CrossRef CAS PubMed.
- P. Broz and V. M. Dixit, Inflammasomes: mechanism of assembly, regulation and signalling, Nat. Rev. Immunol., 2016, 16(7), 407–420 CrossRef CAS PubMed.
- X. Tao, et al., A tandem activation of NLRP3 inflammasome induced by copper oxide nanoparticles and dissolved copper ion in J774A.1 macrophage, J. Hazard. Mater., 2021, 411, 125134 CrossRef CAS PubMed.
- J. C. Simard, et al., Silver nanoparticles induce degradation of the endoplasmic reticulum stress sensor activating transcription factor-6 leading to activation of the NLRP-3 inflammasome, J. Biol. Chem., 2015, 290(9), 5926–5939 CrossRef CAS PubMed.
- A. Murphy, et al., Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes, J. Appl. Toxicol., 2016, 36(10), 1311–1320 CrossRef CAS PubMed.
- M. Zhu, et al., Cell-Penetrating Nanoparticles Activate the Inflammasome to Enhance Antibody Production by Targeting Microtubule-Associated Protein 1-Light Chain 3 for Degradation, ACS Nano, 2020, 14(3), 3703–3717 CrossRef CAS PubMed.
- B. G. Kim, et al., Effect of TiO2 Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness, Allergy, Asthma Immunol. Res., 2017, 9(3), 257–264 CrossRef CAS PubMed.
- E. J. Park, et al., A higher aspect ratio enhanced bioaccumulation and altered immune responses due to intravenously-injected aluminum oxide nanoparticles, J. Immunotoxicol., 2016, 13(4), 439–448 CrossRef CAS PubMed.
- L. Zhang, et al., Investigation of Cytotoxicity, Oxidative Stress, and Inflammatory Responses of Tantalum Nanoparticles in THP-1-Derived Macrophages, Mediators Inflammation, 2020, 2020, 3824593 Search PubMed.
- J. Lugrin, et al., The role of oxidative stress during inflammatory processes, Biol. Chem., 2014, 395(2), 203–230 CrossRef CAS PubMed.
- A. Nel, et al., Toxic potential of materials at the nanolevel, Science, 2006, 311(5761), 622–627 CrossRef CAS PubMed.
- G. Huang, L. Z. Shi and H. Chi, Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination, Cytokine, 2009, 48(3), 161–169 CrossRef CAS PubMed.
- Z. Yuan, et al., Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/p38 MAPK Signaling, Biomolecules, 2019, 9(10), 559 CrossRef CAS PubMed.
- K. Ito, et al., Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells, Nat. Med., 2006, 12(4), 446–451 CrossRef CAS PubMed.
- Y. Yang, et al., Gold nanoparticles synergize with bacterial lipopolysaccharide to enhance class A scavenger receptor dependent particle uptake in neutrophils and augment neutrophil extracellular traps formation, Ecotoxicol. Environ. Saf., 2021, 211, 111900 CrossRef CAS PubMed.
- V. Mulens-Arias, et al., Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics, Biomaterials, 2015, 52, 494–506 CrossRef CAS PubMed.
- L. H. Fell, et al., Impact of individual intravenous iron preparations on the differentiation of monocytes towards macrophages and dendritic cells, Nephrol., Dial., Transplant., 2016, 31(11), 1835–1845 CrossRef CAS PubMed.
- V. A. Senapati, et al., ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: A mechanistic approach, Food Chem. Toxicol., 2015, 85, 61–70 CrossRef CAS PubMed.
- M. Dhupal, et al., Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation, Int. J. Nanomed., 2018, 13, 6735–6750 CrossRef CAS PubMed.
- F. Hong, et al., Immunotoxic effects of thymus in mice following exposure to nanoparticulate TiO(2), Environ. Toxicol., 2017, 32(10), 2234–2243 CrossRef CAS PubMed.
- F. He, X. Ru and T. Wen, NRF2, a Transcription Factor for Stress Response and Beyond, Int. J. Mol. Sci., 2020, 21(13), 4777 CrossRef CAS PubMed.
- I. Buendia, et al., Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases, Pharmacol. Ther., 2016, 157, 84–104 CrossRef CAS PubMed.
- L. Böhmert, et al., Molecular mechanism of silver nanoparticles in human intestinal cells, Nanotoxicology, 2015, 9(7), 852–860 CrossRef PubMed.
- A. Goldstein, et al., The bright side of plasmonic gold nanoparticles; activation of Nrf2, the cellular protective pathway, Nanoscale, 2016, 8(22), 11748–11759 RSC.
- L. Zhang, et al., Stabilization of Nrf2 leading to HO-1 activation protects against zinc oxide nanoparticles-induced endothelial cell death, Nanotoxicology, 2021, 15(6), 779–797 CrossRef CAS PubMed.
- A. Loboda, et al., Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism, Cell. Mol. Life Sci., 2016, 73(17), 3221–3247 CrossRef CAS PubMed.
- J. Liu, et al., Sub-10 nm monoclinic Gd2O3:Eu3+ nanoparticles as dual-modal nanoprobes for magnetic resonance and fluorescence imaging, Langmuir, 2014, 30(43), 13005–13013 CrossRef CAS PubMed.
- J. Wang, et al., Nano-titanium nitride causes developmental toxicity in zebrafish through oxidative stress, Drug Chem. Toxicol., 2022, 45(4), 1660–1669 CrossRef CAS PubMed.
- S. Gui, et al., Renal injury and Nrf2 modulation in mouse kidney following chronic exposure to TiO2 nanoparticles, J. Agric. Food Chem., 2013, 61(37), 8959–8968 CrossRef CAS PubMed.
- E. E. Khayal, et al., Combined lead and zinc oxide-nanoparticles induced thyroid toxicity through 8-OHdG oxidative stress-mediated inflammation, apoptosis, and Nrf2 activation in rats, Environ. Toxicol., 2021, 36(12), 2589–2604 CrossRef CAS PubMed.
- R. Sehsah, et al., Protective role of Nrf2 in zinc oxide nanoparticles-induced lung inflammation in female mice and sexual dimorphism in susceptibility, Toxicol. Lett., 2022, 370, 24–34 CrossRef CAS PubMed.
- Z. Gholinejad, M. H. Khadem Ansari and Y. Rasmi, Titanium dioxide nanoparticles induce endothelial cell apoptosis via cell membrane oxidative damage and p38, PI3K/Akt, NF-κB signaling pathways modulation, J. Trace Elem. Med. Biol., 2019, 54, 27–35 CrossRef CAS PubMed.
- M. D. Mauricio, et al., Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress, Oxid. Med. Cell. Longevity, 2018, 2018, 6231482 CAS.
- G. Ciofani, et al., Effects of cerium oxide nanoparticles on PC12 neuronal-like cells: proliferation, differentiation, and dopamine secretion, Pharm. Res., 2013, 30(8), 2133–2145 CrossRef CAS PubMed.
- C. Zheng, et al., In vivo immunotoxicity of Gd(2) O(3) :Eu(3+) nanoparticles and the associated molecular mechanism, J. Biochem. Mol. Toxicol., 2020, 34(11), e22562 CrossRef CAS PubMed.
- Z. Xie and D. J. Klionsky, Autophagosome formation: core machinery and adaptations, Nat. Cell Biol., 2007, 9(10), 1102–1109 CrossRef CAS PubMed.
- A. G. Renehan, C. Booth and C. S. Potten, What is apoptosis, and why is it important?, Br. Med. J., 2001, 322(7301), 1536–1538 CrossRef CAS PubMed.
- S. Chatterjee, S. Sarkar and S. Bhattacharya, Toxic metals and autophagy, Chem. Res. Toxicol., 2014, 27(11), 1887–1900 Search PubMed.
- B. M. Johnson, et al., Acute exposure to ZnO nanoparticles induces autophagic immune cell death, Nanotoxicology, 2015, 9(6), 737–748 CrossRef CAS PubMed.
- C. H. Jung, et al., mTOR regulation of autophagy, FEBS Lett., 2010, 584(7), 1287–1295 CrossRef CAS PubMed.
- W. J. Song, et al., Zinc Oxide Nanoparticles Induce Autophagy and Apoptosis via Oxidative Injury and Pro-Inflammatory Cytokines in Primary Astrocyte Cultures, Nanomaterials, 2019, 9(7), 1043 CrossRef CAS PubMed.
- W. S. Cho, et al., Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes, Part. Fibre Toxicol., 2011, 8, 27 CrossRef CAS PubMed.
- Y. Chen, et al., Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO(2) Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy, Int. J. Nanomed., 2020, 15, 2011–2026 CrossRef CAS PubMed.
- X. Ma, et al., Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment, ACS Nano, 2011, 5(11), 8629–8639 CrossRef CAS PubMed.
- H. Zhou, et al., Gold nanoparticles impair autophagy flux through shape-dependent endocytosis and lysosomal dysfunction, J. Mater. Chem. B, 2018, 6(48), 8127–8136 RSC.
- R. Jin, et al., Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling, Biomaterials, 2019, 203, 23–30 CrossRef CAS PubMed.
- J. Du, et al., Reduction of polyethylenimine-coated iron oxide nanoparticles induced autophagy and cytotoxicity by lactosylation, Regener. Biomater., 2016, 3(4), 223–229 CrossRef CAS PubMed.
- T. Shen, et al., Lactosylated N-Alkyl polyethylenimine coated iron oxide nanoparticles induced autophagy in mouse dendritic cells, Regener. Biomater., 2018, 5(3), 141–149 CrossRef CAS PubMed.
- J. Zhang, et al., Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells, Cell Death Dis., 2017, 8(7), e2954 CrossRef CAS PubMed.
- Y. R. Lin, et al., Remote Magnetic Control of Autophagy in Mouse B-Lymphoma Cells with Iron Oxide Nanoparticles, Nanomaterials, 2019, 9(4), 551 CrossRef CAS PubMed.
- S. Elmore, Apoptosis: a review of programmed cell death, Toxicol. Pathol., 2007, 35(4), 495–516 CrossRef CAS PubMed.
- S. M. Man and T. D. Kanneganti, Converging roles of caspases in inflammasome activation, cell death and innate immunity, Nat. Rev. Immunol., 2016, 16(1), 7–21 CrossRef CAS PubMed.
- L. Wang, et al., Zinc oxide nanoparticles induce human tenon fibroblast apoptosis through reactive oxygen species and caspase signaling pathway, Arch. Biochem. Biophys., 2020, 683, 108324 CrossRef CAS PubMed.
- H. Attia, H. Nounou and M. Shalaby, Zinc Oxide Nanoparticles Induced Oxidative DNA Damage, Inflammation and Apoptosis in Rat’s Brain after Oral Exposure, Toxics, 2018, 6(2), 29 CrossRef PubMed.
- L. Zhang, et al., Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice, Int. J. Nanomed., 2018, 13, 777–789 CrossRef CAS PubMed.
- Q. He, et al., Titanium dioxide nanoparticles induce mouse hippocampal neuron apoptosis via oxidative stress- and calcium imbalance-mediated endoplasmic reticulum stress, Environ. Toxicol. Pharmacol., 2018, 63, 6–15 CrossRef CAS PubMed.
- H. Liu, et al., Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell, J. Hazard. Mater., 2021, 401, 123349 CrossRef CAS PubMed.
- Q. Wu, et al., Iron oxide nanoparticles and induced autophagy in human monocytes, Int. J. Nanomed., 2017, 12, 3993–4005 CrossRef CAS PubMed.
- D. Maysinger, et al., Gold nanoclusters elicit homeostatic perturbations in glioblastoma cells and adaptive changes of lysosomes, Theranostics, 2020, 10(4), 1633–1648 CrossRef CAS PubMed.
- Y. Xu, et al., Silver nanoparticles impede phorbol myristate acetate-induced monocyte-macrophage differentiation and autophagy, Nanoscale, 2015, 7(38), 16100–16109 RSC.
- R. F. Hamilton, et al., Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity, Part. Fibre Toxicol., 2009, 6, 35 CrossRef PubMed.
- A. Shah, et al., Feraheme® suppresses immune function of human T lymphocytes through mitochondrial damage and mitoROS production, Toxicol. Appl. Pharmacol., 2018, 350, 52–63 CrossRef CAS PubMed.
- T. G. Zhang, et al., Impairment of mitochondrial dynamics involved in iron oxide nanoparticle-induced dysfunction of dendritic cells was alleviated by autophagy inhibitor 3-methyladenine, J. Appl. Toxicol., 2020, 40(5), 631–642 CrossRef CAS PubMed.
- P. Orlowski, et al., Tannic Acid-Modified Silver and Gold Nanoparticles as Novel Stimulators of Dendritic Cells Activation, Front. Immunol., 2018, 9, 1115 CrossRef PubMed.
- A. K. Dey, et al., Impact of Gold Nanoparticles on the Functions of Macrophages and Dendritic Cells, Cells, 2021, 10(1), 96 CrossRef CAS PubMed.
- A. A. Khan, et al., Endoplasmic Reticulum Stress Provocation by Different Nanoparticles: An Innovative Approach to Manage the Cancer and Other Common Diseases, Molecules, 2020, 25(22), 5336 CrossRef CAS PubMed.
- H. Yasui, et al., Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles, Cancer Lett., 2014, 347(1), 151–158 CrossRef CAS PubMed.
- E. J. Park, et al., Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and ER stress in RAW264.7 cells, Toxicol. in Vitro, 2014, 28(8), 1402–1412 CrossRef CAS PubMed.
- B. Ghaemi, et al., Supramolecular Insights into Domino Effects of Ag@ZnO-Induced Oxidative Stress in Melanoma Cancer Cells, ACS Appl. Mater. Interfaces, 2019, 11(50), 46408–46418 CrossRef CAS PubMed.
- X. Ma, et al., Evaluation of Turning-Sized Gold Nanoparticles on Cellular Adhesion by Golgi Disruption in Vitro and in Vivo, Nano Lett., 2019, 19(12), 8476–8487 CrossRef CAS PubMed.
- D. M. Pegtel and S. J. Gould, Exosomes, Annu. Rev. Biochem., 2019, 88, 487–514 CrossRef CAS PubMed.
- M. Zhu, et al., Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation, Small, 2012, 8(3), 404–412 CrossRef CAS PubMed.
- M. Kumari, A. Mukherjee and N. Chandrasekaran, Genotoxicity of silver nanoparticles in Allium cepa, Sci. Total Environ., 2009, 407(19), 5243–5246 CrossRef CAS PubMed.
- J. Yu, et al., Insights into the epigenetic effects of nanomaterials on cells, Biomater. Sci., 2020, 8(3), 763–775 RSC.
- M. Ghosh, L. Godderis and P. Hoet, Epigenetic Mechanisms in Understanding Nanomaterial-Induced Toxicity, Adv. Exp. Med. Biol., 2022, 1357, 195–223 CrossRef PubMed.
- Z. Magdolenova, et al., Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles, Nanotoxicology, 2014, 8(3), 233–278 CrossRef CAS PubMed.
- M. Dusinska, et al., Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing?, Food Chem. Toxicol., 2017, 109(Pt 1), 797–811 CrossRef CAS PubMed.
- W. Zhang, et al., Engineered nanoparticle-induced epigenetic changes: An important consideration in nanomedicine, Acta Biomater., 2020, 117, 93–107 CrossRef CAS PubMed.
- K. S. Butler, et al., Silver nanoparticles: correlating nanoparticle size and cellular uptake with genotoxicity, Mutagenesis, 2015, 30(4), 577–591 CrossRef CAS PubMed.
- Y. Li, et al., Differential genotoxicity mechanisms of silver nanoparticles and silver ions, Arch. Toxicol., 2017, 91(1), 509–519 CrossRef CAS PubMed.
- Q. Xia, et al., The effect of particle size on the genotoxicity of gold nanoparticles, J. Biomed. Mater. Res., Part A, 2017, 105(3), 710–719 CrossRef CAS PubMed.
- Z. Magdolenova, et al., Coating-dependent induction of cytotoxicity and genotoxicity of iron oxide nanoparticles, Nanotoxicology, 2015, 9(Suppl 1), 44–56 CrossRef CAS PubMed.
- S. Ghosh, et al., Genotoxicity and biocompatibility of superparamagnetic iron oxide nanoparticles: Influence of surface modification on biodistribution, retention, DNA damage and oxidative stress, Food Chem. Toxicol., 2020, 136, 110989 CrossRef CAS PubMed.
- D. Couto, et al., Polyacrylic acid coated and non-coated iron oxide nanoparticles are not genotoxic to human T lymphocytes, Toxicol. Lett., 2015, 234(2), 67–73 CrossRef CAS PubMed.
- H. Jiang, et al., Effects of cobalt nanoparticles on human T cells in vitro, Biol. Trace Elem. Res., 2012, 146(1), 23–29 CrossRef CAS PubMed.
- S. Rajiv, et al., Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron(III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro, Hum. Exp. Toxicol., 2016, 35(2), 170–183 CrossRef CAS PubMed.
- T. Chen, J. Yan and Y. Li, Genotoxicity of titanium dioxide nanoparticles, J. Food Drug Anal., 2014, 22(1), 95–104 CrossRef CAS PubMed.
- A. Kazimirova, et al., Titanium dioxide nanoparticles tested for genotoxicity with the comet and micronucleus assays in vitro, ex vivo and in vivo, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2019, 843, 57–65 CrossRef CAS PubMed.
- A. Sliwinska, et al., Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles, Toxicol. Mech. Methods, 2015, 25(3), 176–183 CrossRef CAS PubMed.
- Y. Teow, et al., Health impact and safety of engineered nanomaterials, Chem. Commun., 2011, 47(25), 7025–7038 RSC.
- C. Dupont, D. R. Armant and C. A. Brenner, Epigenetics: definition, mechanisms and clinical perspective, Semin. Reprod. Med., 2009, 27(5), 351–357 CrossRef CAS PubMed.
- H. J. Eom, et al., Integrated mRNA and micro RNA profiling reveals epigenetic mechanism of differential sensitivity of Jurkat T cells to AgNPs and Ag ions, Toxicol. Lett., 2014, 229(1), 311–318 CrossRef CAS PubMed.
- Y. Qian, et al., Silver nanoparticle-induced hemoglobin decrease involves alteration of histone 3 methylation status, Biomaterials, 2015, 70, 12–22 CrossRef CAS PubMed.
- A. M. Tabish, et al., Changes in DNA Methylation in Mouse Lungs after a Single Intra-Tracheal Administration of Nanomaterials, PLoS One, 2017, 12(1), e0169886 CrossRef PubMed.
- A. Stoccoro, et al., Epigenetic effects of nano-sized materials, Toxicology, 2013, 313(1), 3–14 CrossRef CAS PubMed.
- J. Ndika, et al., Silver, titanium dioxide, and zinc oxide nanoparticles trigger miRNA/isomiR expression changes in THP-1 cells that are proportional to their health hazard potential, Nanotoxicology, 2019, 13(10), 1380–1395 CrossRef CAS PubMed.
- X. Lu, et al., Short-term exposure to engineered nanomaterials affects cellular epigenome, Nanotoxicology, 2016, 10(2), 140–150 CAS.
- X. Lu, et al., In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles, Nanotoxicology, 2016, 10(5), 629–639 CrossRef CAS PubMed.
- T. A. Ngobili and M. A. Daniele, Nanoparticles and direct immunosuppression, Exp. Biol. Med., 2016, 241(10), 1064–1073 CrossRef CAS PubMed.
- A. N. Yilma, et al., Anti-inflammatory effects of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles in mouse macrophages infected with live Chlamydia trachomatis, Int. J. Nanomed., 2013, 8, 2421–2432 Search PubMed.
- C. C. Shen, et al., A single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c mice, Int. J. Nanomed., 2011, 6, 1229–1235 CAS.
- Y. P. Hsiao, et al., Iron oxide nanoparticles attenuate T helper 17 cell responses in vitro and in vivo, Int. Immunopharmacol., 2018, 58, 32–39 CrossRef CAS PubMed.
- S. M. Hirst, et al., Anti-inflammatory properties of cerium oxide nanoparticles, Small, 2009, 5(24), 2848–2856 CrossRef CAS PubMed.
- A. E. Palmer and K. J. Franz, Introduction to “cellular metal homeostasis and trafficking”, Chem. Rev., 2009, 109(10), 4533–4535 CrossRef CAS PubMed.
- M. A. Zoroddu, et al., The essential metals for humans: a brief overview, J. Inorg. Biochem., 2019, 195, 120–129 CrossRef CAS PubMed.
- S. Ni, et al., Iron Metabolism and Immune Regulation, Front. Immunol., 2022, 13, 816282 CrossRef CAS PubMed.
- M. Chevallet, et al., Impact of labile metal nanoparticles on cellular homeostasis. Current developments in imaging, synthesis and applications, Biochim. Biophys. Acta, Gen. Subj., 2017, 1861(6), 1566–1577 CrossRef CAS PubMed.
- T. Xia, et al., Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties, ACS Nano, 2008, 2(10), 2121–2134 CrossRef CAS PubMed.
- M. Cuillel, et al., Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions, Nanoscale, 2014, 6(3), 1707–1715 RSC.
- G. K. Andrews, Cellular zinc sensors: MTF-1 regulation of gene expression, BioMetals, 2001, 14(3–4), 223–237 CrossRef CAS PubMed.
- J. Bourquin, et al., Biodistribution, Clearance, and Long-Term Fate of Clinically Relevant Nanomaterials, Adv. Mater., 2018, 30(19), e1704307 CrossRef PubMed.
- A. Zamborlin, et al., The Fate of Intranasally Instilled Silver Nanoarchitectures, Nano Lett., 2022, 22(13), 5269–5276 CrossRef CAS PubMed.
- M. Chanana, et al., Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion, Angew. Chem., Int. Ed., 2013, 52(15), 4179–4183 CrossRef CAS PubMed.
- S. J. Soenen, et al., (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications, Chem. Rev., 2015, 115(5), 2109–2135 CrossRef CAS PubMed.
- G. Nichols, et al., A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization, J. Pharm. Sci., 2002, 91(10), 2103–2109 CrossRef CAS PubMed.
- S. Keshavan, et al., Nano-bio interactions: a neutrophil-centric view, Cell Death Dis., 2019, 10(8), 569 CrossRef PubMed.
- Q. Feng, et al., Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings, Sci. Rep., 2018, 8(1), 2082 CrossRef PubMed.
- J. P. Almeida, et al., In vivo immune cell distribution of gold nanoparticles in naïve and tumor bearing mice, Small, 2014, 10(4), 812–819 CrossRef CAS PubMed.
- D. M. Smith, J. K. Simon and J. R. Baker Jr., Applications of nanotechnology for immunology, Nat. Rev. Immunol., 2013, 13(8), 592–605 CrossRef CAS PubMed.
- D. P. Lankveld, et al., The kinetics of the tissue distribution of silver nanoparticles of different sizes, Biomaterials, 2010, 31(32), 8350–8361 CrossRef CAS PubMed.
- M. Yu and J. Zheng, Clearance Pathways and Tumor Targeting of Imaging Nanoparticles, ACS Nano, 2015, 9(7), 6655–6674 CrossRef CAS PubMed.
- G. Yang, et al., Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications, Adv. Mater., 2019, 31(10), e1805730 CrossRef PubMed.
- D. J. McClements, H. Xiao and P. Demokritou, Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles, Adv. Colloid Interface Sci., 2017, 246, 165–180 CrossRef CAS PubMed.
- J. Kolosnjaj-Tabi, J. Volatron and F. Gazeau, Basic Principles of In Vivo Distribution, Toxicity, and Degradation of Prospective Inorganic Nanoparticles for Imaging, Springer International Publishing, 2017 Search PubMed.
- Z. Cai, et al., Hyaluronan-Inorganic Nanohybrid Materials for Biomedical Applications, Biomacromolecules, 2017, 18(6), 1677–1696 CrossRef CAS PubMed.
- J. Y. Wang, et al., Effects of surface charges of gold nanoclusters on long-term in vivo biodistribution, toxicity, and cancer radiation therapy, Int. J. Nanomed., 2016, 11, 3475–3485 CrossRef CAS PubMed.
- W. H. De Jong, et al., Particle size-dependent organ distribution of gold nanoparticles after intravenous administration, Biomaterials, 2008, 29(12), 1912–1919 CrossRef CAS PubMed.
- H. Tang, et al., Blood Clearance, Distribution, Transformation, Excretion, and Toxicity of Near-Infrared Quantum Dots Ag2Se in Mice, ACS Appl. Mater. Interfaces, 2016, 8(28), 17859–17869 CrossRef CAS PubMed.
- P. Falagan-Lotsch, E. M. Grzincic and C. J. Murphy, One low-dose exposure of gold nanoparticles induces long-term changes in human cells, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(47), 13318–13323 CrossRef CAS PubMed.
- J. Unnithan, et al., Aqueous synthesis and concentration-dependent dermal toxicity of TiO2 nanoparticles in Wistar rats, Biol. Trace Elem. Res., 2011, 143(3), 1682–1694 CrossRef CAS PubMed.
- A. Spengler, L. Wanninger and S. Pflugmacher, Oxidative stress mediated toxicity of TiO(2) nanoparticles after a concentration and time dependent exposure of the aquatic macrophyte Hydrilla verticillata, Aquat. Toxicol., 2017, 190, 32–39 CrossRef CAS PubMed.
- H. Guo, et al., Intravenous administration of silver nanoparticles causes organ toxicity through intracellular ROS-related loss of inter-endothelial junction, Part. Fibre Toxicol., 2016, 13, 21 CrossRef PubMed.
- J. Choi, et al., Toxicity of zinc oxide nanoparticles in rats treated by two different routes: single intravenous injection and single oral administration, J. Toxicol. Environ. Health, Part A, 2015, 78(4), 226–243 CrossRef CAS PubMed.
- X. Jia, et al., The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice, Nanoscale Res. Lett., 2017, 12(1), 478 CrossRef PubMed.
- B. Shahare and M. Yashpal, Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice, Toxicol. Mech. Methods, 2013, 23(3), 161–167 CrossRef CAS PubMed.
- C. Recordati, et al., Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects, Part. Fibre Toxicol., 2016, 13, 12 CrossRef PubMed.
- Y. Yamagishi, et al., Acute and chronic nephrotoxicity of platinum nanoparticles in mice, Nanoscale Res. Lett., 2013, 8(1), 395 CrossRef PubMed.
- E. Sadauskas, et al., Protracted elimination of gold nanoparticles from mouse liver, Nanomedicine, 2009, 5(2), 162–169 CrossRef CAS PubMed.
- S. Dragoni, et al., Gold nanoparticles uptake and cytotoxicity assessed on rat liver precision-cut slices, Toxicol. Sci., 2012, 128(1), 186–197 CrossRef CAS PubMed.
- E. J. Pearce and E. L. Pearce, Immunometabolism in 2017: Driving immunity: all roads lead to metabolism, Nat. Rev. Immunol., 2018, 18(2), 81–82 CrossRef CAS PubMed.
- E. L. Pearce and E. J. Pearce, Metabolic pathways in immune cell activation and quiescence, Immunity, 2013, 38(4), 633–643 CrossRef CAS PubMed.
- K. Voss, et al., A guide to interrogating immunometabolism, Nat. Rev. Immunol., 2021, 21(10), 637–652 CrossRef CAS PubMed.
- Y. Hu, et al., mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP, Glia, 2020, 68(5), 1031–1045 CrossRef PubMed.
- M. Fumagalli, et al., How to reprogram microglia toward beneficial functions, Glia, 2018, 66(12), 2531–2549 CrossRef PubMed.
- S. K. Biswas and A. Mantovani, Orchestration of metabolism by macrophages, Cell Metab., 2012, 15(4), 432–437 CrossRef CAS PubMed.
- C. S. Nascimento, et al., Immunotherapy for cancer: effects of iron oxide nanoparticles on polarization of tumor-associated macrophages, Nanomedicine, 2021, 16(29), 2633–2650 CrossRef CAS PubMed.
- M. W. Hentze, et al., Two to tango: regulation of Mammalian iron metabolism, Cell, 2010, 142(1), 24–38 CrossRef CAS PubMed.
- L. He, et al., The role of morphology, shell composition and protein corona formation in Au/Fe(3)O(4) composite nanoparticle mediated macrophage responses, J. Mater. Chem. B, 2021, 9(32), 6387–6395 RSC.
- R. Tiwari, et al., Perinatal exposure to silver nanoparticles reprograms immunometabolism and promotes pancreatic beta-cell death and kidney damage in mice, Nanotoxicology, 2021, 15(5), 636–660 CrossRef CAS PubMed.
- R. Tiwari, et al., Gestational exposure to silver nanoparticles enhances immune adaptation and protection against streptozotocin-induced diabetic nephropathy in mice offspring, Nanotoxicology, 2022, 16(4), 450–471 CrossRef CAS PubMed.
- I. Mikelez-Alonso, et al., Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: A look into how nanoparticles enhance NK cell activity, Adv. Drug Delivery Rev., 2021, 176, 113860 CrossRef CAS PubMed.
- A. C. Anselmo and S. Mitragotri, Nanoparticles in the clinic: An update, Bioeng. Transl. Med., 2019, 4(3), e10143 Search PubMed.
- F. Wang, et al., Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation, Cell Metab., 2018, 28(3), 463–475.e4 CrossRef CAS PubMed.
- N. J. MacIver, R. D. Michalek and J. C. Rathmell, Metabolic regulation of T lymphocytes, Annu. Rev. Immunol., 2013, 31, 259–283 CrossRef CAS PubMed.
- R. Wang and D. R. Green, Metabolic checkpoints in activated T cells, Nat. Immunol., 2012, 13(10), 907–915 CrossRef CAS PubMed.
- K. N. Pollizzi and J. D. Powell, Integrating canonical and metabolic signalling programmes in the regulation of T cell responses, Nat. Rev. Immunol., 2014, 14(7), 435–446 CrossRef CAS PubMed.
- H. Kojima, et al., Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine B cell development in bone marrow, J. Immunol., 2010, 184(1), 154–163 CrossRef CAS PubMed.
- C. A. Doughty, et al., Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth, Blood, 2006, 107(11), 4458–4465 CrossRef CAS PubMed.
- F. J. Dufort, et al., Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism, J. Immunol., 2007, 179(8), 4953–4957 CrossRef CAS PubMed.
- B. Everts, et al., TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation, Nat. Immunol., 2014, 15(4), 323–332 CrossRef CAS PubMed.
- C. M. Krawczyk, et al., Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation, Blood, 2010, 115(23), 4742–4749 CrossRef CAS PubMed.
- B. Everts, et al., Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells, Blood, 2012, 120(7), 1422–1431 CrossRef CAS PubMed.
- J. I. Odegaard and A. Chawla, Alternative macrophage activation and metabolism, Annu. Rev. Pathol., 2011, 6, 275–297 CrossRef CAS PubMed.
- D. Vats, et al., Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation, Cell Metab., 2006, 4(1), 13–24 CrossRef CAS PubMed.
- B. J. van Raam, A. J. Verhoeven and T. W. Kuijpers, Mitochondria in neutrophil apoptosis, Int. J. Hematol., 2006, 84(3), 199–204 CrossRef CAS PubMed.
- D. C. Dale, L. Boxer and W. C. Liles, The phagocytes: neutrophils and monocytes, Blood, 2008, 112(4), 935–945 CrossRef CAS PubMed.
- H. Park, et al., Metabolic regulator Fnip1 is crucial for iNKT lymphocyte development, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(19), 7066–7071 CrossRef CAS PubMed.
- M. Dose, et al., Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(21), 8641–8646 CrossRef CAS PubMed.
- J. Henao-Mejia, et al., The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis, Immunity, 2013, 38(5), 984–997 CrossRef CAS PubMed.
- N. Lee, et al., Magnetosome-like ferrimagnetic iron oxide nanocubes for highly sensitive MRI of single cells and transplanted pancreatic islets, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(7), 2662–2667 CrossRef CAS PubMed.
- J. Xie, et al., Human serum albumin coated iron oxide nanoparticles for efficient cell labeling, Chem. Commun., 2010, 46(3), 433–435 RSC.
- H. Maeda, H. Nakamura and J. Fang, The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo, Adv. Drug Delivery Rev., 2013, 65(1), 71–79 CrossRef CAS PubMed.
- A. J. Cole, V. C. Yang and A. E. David, Cancer theranostics: the rise of targeted magnetic nanoparticles, Trends Biotechnol., 2011, 29(7), 323–332 CrossRef CAS PubMed.
- Y. Hu, et al., Multifunctional Fe3O4@Au core/shell nanostars: a unique platform for multimode imaging and photothermal therapy of tumors, Sci. Rep., 2016, 6, 28325 CrossRef CAS PubMed.
- Y. H. Hwang, M. J. Kim and D. Y. Lee, MRI-sensitive contrast agent with anticoagulant activity for surface camouflage of transplanted pancreatic islets, Biomaterials, 2017, 138, 121–130 CrossRef CAS PubMed.
- Z. Wang and A. Cuschieri, Tumour cell labelling by magnetic nanoparticles with determination of intracellular iron content and spatial distribution of the intracellular iron, Int. J. Mol. Sci., 2013, 14(5), 9111–9125 CrossRef PubMed.
- H. Xu, et al., Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood, Biomaterials, 2011, 32(36), 9758–9765 CrossRef CAS PubMed.
- J. Yao, et al., ROS scavenging Mn(3)O(4) nanozymes for in vivo anti-inflammation, Chem. Sci., 2018, 9(11), 2927–2933 RSC.
- X. Jiang, et al., Crossover between anti- and pro-oxidant activities of different manganese oxide nanoparticles and their biological implications, J. Mater. Chem. B, 2020, 8(6), 1191–1201 RSC.
- Y. Zhang, et al., Multienzymatic Antioxidant Activity of Manganese-Based Nanoparticles for Protection against Oxidative Cell Damage, ACS Biomater. Sci. Eng., 2022, 8(2), 638–648 CrossRef CAS PubMed.
- E. Hoseinzadeh, et al., A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms, Curr. Drug Metab., 2017, 18(2), 120–128 CrossRef CAS PubMed.
- H. S. Gill, et al., Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty, Trends Mol. Med., 2012, 18(3), 145–155 CrossRef CAS PubMed.
- R. H. Kim, D. A. Dennis and J. T. Carothers, Metal-on-metal total hip arthroplasty, J. Arthroplasty, 2008, 23(7 Suppl), 44–46 Search PubMed.
- G. P. Jose, et al., Singlet oxygen mediated DNA degradation by copper nanoparticles: potential towards cytotoxic effect on cancer cells, J. Nanobiotechnol., 2011, 9, 9 CrossRef CAS PubMed.
- X. Ma, et al., Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review, Front. Surg., 2022, 9, 905892 CrossRef PubMed.
- M. A. Shahbazi, et al., The versatile biomedical applications of bismuth-based nanoparticles and composites: therapeutic, diagnostic, biosensing, and regenerative properties, Chem. Soc. Rev., 2020, 49(4), 1253–1321 RSC.
- Z. Li, et al., Dual-Stimuli Responsive Bismuth Nanoraspberries for Multimodal Imaging and Combined Cancer Therapy, Nano Lett., 2018, 18(11), 6778–6788 CrossRef CAS PubMed.
- A. Naghizadeh, S. Mohammadi-Aghdam and S. Mortazavi-Derazkola, Novel CoFe(2)O(4)@ZnO-CeO(2) ternary nanocomposite: Sonochemical green synthesis using Crataegus microphylla extract, characterization and their application in catalytic and antibacterial activities, Bioorg. Chem., 2020, 103, 104194 CrossRef CAS PubMed.
- G. Tortella, et al., Bactericidal and Virucidal Activities of Biogenic Metal-Based Nanoparticles: Advances and Perspectives, Antibiotics, 2021, 10(7), 783 CrossRef CAS PubMed.
- A. Goel, Boydston, et al.: The Impact of Alternative Alkalinizing Agents on 24-Hour Urine Parameters (Urology 2020 Apr 21;S0090-4295(20)30428-3.), Urology, 2020, 143, 270–271, DOI:10.1016/j.urology.2020.04.047.
- S. R. Alizadeh and M. A. Ebrahimzadeh, Characterization and Anticancer Activities of Green Synthesized CuO Nanoparticles, A Review, Anti-Cancer Agents Med. Chem., 2021, 21(12), 1529–1543 CrossRef CAS PubMed.
- L. Shkodenko, I. Kassirov and E. Koshel, Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations, Microorganisms, 2020, 8(10), 1545 CrossRef CAS PubMed.
- B. Xu, et al., Synthesis, Characterization, and Antifogging Application of Polymer/Al2O3 Nanocomposite Hydrogels with High Strength and Self-Healing Capacity, Polymers, 2018, 10(12), 1362 CrossRef PubMed.
- V. Iribarnegaray, et al., Magnesium-doped zinc oxide nanoparticles alter biofilm formation of Proteus mirabilis, Nanomedicine, 2019, 14(12), 1551–1564 CrossRef CAS PubMed.
- A. Aghebati-Maleki, et al., Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers, J. Cell Physiol., 2020, 235(3), 1962–1972 CrossRef CAS PubMed.
- K. Jadhav, et al., Phytosynthesis of gold nanoparticles: Characterization, biocompatibility, and evaluation of its osteoinductive potential for application in implant dentistry, Mater. Sci. Eng., C, 2018, 93, 664–670 CrossRef CAS PubMed.
- J. Noonan, et al., In vivo multiplex molecular imaging of vascular inflammation using surface-enhanced Raman spectroscopy, Theranostics, 2018, 8(22), 6195–6209 CrossRef CAS PubMed.
- S. C. Boca, et al., Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy, Cancer Lett., 2011, 311(2), 131–140 CrossRef CAS PubMed.
- J. Liu, et al., TAT-modified nanosilver for combating multidrug-resistant cancer, Biomaterials, 2012, 33(26), 6155–6161 CrossRef CAS PubMed.
- L. S. Abebe, et al., Point-of-Use Removal of Cryptosporidium parvum from Water: Independent Effects of Disinfection by Silver Nanoparticles and Silver Ions and by Physical Filtration in Ceramic Porous Media, Environ. Sci. Technol., 2015, 49(21), 12958–12967 CrossRef CAS PubMed.
- H. B. Dias, et al., Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti biofilm filler content for composite resins, Dent. Mater., 2019, 35(2), e36–e46 CrossRef CAS PubMed.
- L. Zhang, et al., Reducing stress on cells with apoferritin-encapsulated platinum nanoparticles, Nano Lett., 2010, 10(1), 219–223 CrossRef PubMed.
- J. S. Lee, et al., Highly Sensitive and Selective Field-Effect-Transistor NonEnzyme Dopamine Sensors Based on Pt/Conducting Polymer Hybrid Nanoparticles, Small, 2015, 11(20), 2399–2406 CrossRef CAS PubMed.
- Z. Bai, et al., Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells, Biosens. Bioelectron., 2016, 82, 185–194 CrossRef CAS PubMed.
- A. Abed, et al., Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles, Front. Pharmacol., 2022, 13, 797804 CrossRef CAS PubMed.
- J. T. Weiss, et al., Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach, Nat. Commun., 2014, 5, 3277 CrossRef PubMed.
- S. Tang, M. Chen and N. Zheng, Sub-10 nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy, Small, 2014, 10(15), 3139–3144 CrossRef CAS PubMed.
- N. Huang, et al., Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research, Biomaterials, 2017, 141, 296–313 CrossRef CAS PubMed.
- Y. Liu, et al., Ru nanoparticles coated with γ-Fe(2)O(3) promoting and monitoring the differentiation of human mesenchymal stem cells via MRI tracking, Colloids Surf., B, 2018, 170, 701–711 CrossRef CAS PubMed.
- F. Xia, et al., Ultrasmall Ruthenium Nanoparticles with Boosted Antioxidant Activity Upregulate Regulatory T Cells for Highly Efficient Liver Injury Therapy, Small, 2022, 18(29), e2201558 CrossRef PubMed.
- S. Kang, et al., Morphology-Controlled Synthesis of Rhodium Nanoparticles for Cancer Phototherapy, ACS Nano, 2018, 12(7), 6997–7008 CrossRef CAS PubMed.
- L. Zhang, et al., AIE Multinuclear Ir(III) Complexes for Biocompatible Organic Nanoparticles with Highly Enhanced Photodynamic Performance, Adv. Sci., 2019, 6(5), 1802050 CrossRef PubMed.
- Y. Fan, et al., pH-activated size reduction of large compound nanoparticles for in vivo nucleus-targeted drug delivery, Biomaterials, 2016, 85, 30–39 CrossRef CAS PubMed.
- Y. Wu, et al., Visible-light-excited and europium-emissive nanoparticles for highly-luminescent bioimaging in vivo, Biomaterials, 2014, 35(22), 5830–5839 CrossRef CAS PubMed.
- M. M. El-Sheekh, et al., Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique, Int. J. Environ. Health Res., 2022, 32(3), 616–627 CrossRef CAS PubMed.
- Y. Wang, et al., Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation, Small, 2021, 17(41), e2101505 CrossRef PubMed.
- R. P. Senthilkumar, et al., Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: As a bionanomaterials, Int. J. Biol. Macromol., 2017, 104(Pt B), 1746–1752 CrossRef CAS PubMed.
- J. Xiang, et al., Cerium Oxide Nanoparticle Modified Scaffold Interface Enhances Vascularization of Bone Grafts by Activating Calcium Channel of Mesenchymal Stem Cells, ACS Appl. Mater. Interfaces, 2016, 8(7), 4489–4499 CrossRef CAS PubMed.
- O. M. David, et al., The Stability and Anti-Angiogenic Properties of Titanium Dioxide Nanoparticles (TiO2NPs) Using Caco-2 Cells, Biomolecules, 2022, 12(10), 1334 CrossRef CAS PubMed.
- S. Çeşmeli and C. Biray Avci, Application of titanium dioxide (TiO(2)) nanoparticles in cancer therapies, J. Drug Targeting, 2019, 27(7), 762–766 CrossRef PubMed.
- T. Wang, et al., Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery, Acta Biomater., 2015, 13, 354–363 CrossRef CAS PubMed.
- X. Wang, et al., Advances in surface modification of tantalum and porous tantalum for rapid osseointegration: A thematic review, Front. Bioeng. Biotechnol., 2022, 10, 983695 CrossRef PubMed.
- A. Rafieerad, et al., Fabrication of Smart Tantalum Carbide MXene Quantum Dots with Intrinsic Immunomodulatory Properties for Treatment of Allograft Vasculopathy, Adv. Funct. Mater., 2021, 31(46), 2106786 CrossRef CAS PubMed.
- U. S. Gaharwar, R. Meena and P. Rajamani, Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes, J. Appl. Toxicol., 2017, 37(10), 1232–1244 CrossRef CAS PubMed.
- O. M. Posada, R. J. Tate and M. H. Grant, Effects of CoCr metal wear debris generated from metal-on-metal hip implants and Co ions on human monocyte-like U937 cells, Toxicol. in Vitro, 2015, 29(2), 271–280 CrossRef CAS PubMed.
- Y. M. Kwon, et al., Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro, Biomed. Mater., 2009, 4(2), 025018 CrossRef PubMed.
- S. L. More, et al., Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles, Nanomaterials, 2021, 11(3), 642 CrossRef CAS PubMed.
- X. Chen and C. Gao, Influences of size and surface coating of gold nanoparticles on inflammatory activation of macrophages, Colloids Surf., B, 2017, 160, 372–380 CrossRef CAS PubMed.
- B. Vuković, et al., Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells, Nanomaterials, 2020, 10(7), 1390 CrossRef PubMed.
- S. Murugadoss, et al., Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo, Part. Fibre Toxicol., 2020, 17(1), 10 CrossRef CAS PubMed.
- X. Zhang, et al., Iron Oxide Nanoparticles Induce Autophagosome Accumulation through Multiple Mechanisms: Lysosome Impairment, Mitochondrial Damage, and ER Stress, Mol. Pharm., 2016, 13(7), 2578–2587 CrossRef CAS PubMed.
- A. Sabareeswaran, et al., Effect of surface-modified superparamagnetic iron oxide nanoparticles (SPIONS) on mast cell infiltration: An acute in vivo study, Nanomedicine, 2016, 12(6), 1523–1533 CrossRef CAS PubMed.
- L. Zha, et al., Chromium(III) nanoparticles affect hormone and immune responses in heat-stressed rats, Biol. Trace Elem. Res., 2009, 129(1–3), 157–169 CrossRef CAS PubMed.
- X. Chang, et al., Role of NF-κB activation and Th1/Th2 imbalance in pulmonary toxicity induced by nano NiO, Environ. Toxicol., 2017, 32(4), 1354–1362 CrossRef CAS PubMed.
- X. Zhou, et al., The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats, Int. J. Mol. Sci., 2019, 20(6), 1469 CrossRef CAS PubMed.
- I. C. Lee, et al., Copper nanoparticles induce early fibrotic changes in the liver via TGF-β/Smad signaling and cause immunosuppressive effects in rats, Nanotoxicology, 2018, 12(6), 637–651 CrossRef CAS PubMed.
- R. Sadiq, et al., Genotoxicity of aluminium oxide, iron oxide, and copper nanoparticles in mouse bone marrow cells, Arh. Hig. Rada Toksikol., 2021, 72(4), 315–325 CAS.
- J. Tulinska, et al., Six-week inhalation of CdO nanoparticles in mice: The effects on immune response, oxidative stress, antioxidative defense, fibrotic response, and bones, Food Chem. Toxicol., 2020, 136, 110954 CrossRef CAS PubMed.
- W. H. De Jong, et al., Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats, Biomaterials, 2013, 34(33), 8333–8343 CrossRef CAS PubMed.
- X. Chang, et al., Effects of Th1 and Th2 cells balance in pulmonary injury induced by nano titanium dioxide, Environ. Toxicol. Pharmacol., 2014, 37(1), 275–283 CrossRef CAS PubMed.
- X. Sang, et al., Immunomodulatory effects in the spleen-injured mice following exposure to titanium dioxide nanoparticles, J. Biomed. Mater. Res., Part A, 2014, 102(10), 3562–3572 CrossRef PubMed.
- W. Mu, et al., Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice, J. Agric. Food Chem., 2019, 67(33), 9382–9389 CrossRef CAS PubMed.
- L. Yao, et al., Oral exposure of titanium oxide nanoparticles induce ileum physical barrier dysfunction via Th1/Th2 imbalance, Environ. Toxicol., 2020, 35(9), 982–990 CrossRef CAS PubMed.
- P. Jalili, et al., Genotoxicity of Aluminum and Aluminum Oxide Nanomaterials in Rats Following Oral Exposure, Nanomaterials, 2020, 10(2), 305 CrossRef CAS PubMed.
- L. Talamini, et al., Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles, ACS Nano, 2017, 11(6), 5519–5529 CrossRef CAS PubMed.
- J. S. Hong, et al., Combined repeated-dose toxicity study of silver nanoparticles with the reproduction/developmental toxicity screening test, Nanotoxicology, 2014, 8(4), 349–362 CrossRef CAS PubMed.
- K. F. Chung, et al., Inactivation, Clearance, and Functional Effects of Lung-Instilled Short and Long Silver Nanowires in Rats, ACS Nano, 2017, 11(3), 2652–2664 CrossRef CAS PubMed.
- R. M. Silva, et al., Aerosolized Silver Nanoparticles in the Rat Lung and Pulmonary Responses over Time, Toxicol. Pathol., 2016, 44(5), 673–686 CrossRef CAS PubMed.
- W. G. Kreyling, et al., Quantitative biokinetics over a 28 day period of freshly generated, pristine, 20 nm silver nanoparticle aerosols in healthy adult rats after a single 1½-hour inhalation exposure, Part. Fibre Toxicol., 2020, 17(1), 21 CrossRef CAS PubMed.
- W. S. Cho, et al., Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100 nm-sized PEG-coated gold nanoparticles, Toxicol. Lett., 2009, 191(1), 96–102 CrossRef CAS PubMed.
- J. H. Ji, et al., Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats, Inhalation Toxicol., 2007, 19(10), 857–871 CrossRef CAS PubMed.
- Y. S. Kim, et al., Subchronic oral toxicity of silver nanoparticles, Part. Fibre Toxicol., 2010, 7, 20 CrossRef PubMed.
- D. B. Warheit, S. C. Brown and E. M. Donner, Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles, Food Chem. Toxicol., 2015, 84, 208–224 CrossRef CAS PubMed.
- K. E. Ibrahim, et al., Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles, Molecules, 2018, 23(8), 1848 CrossRef PubMed.
- S. K. Balasubramanian, et al., Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats, Biomaterials, 2010, 31(8), 2034–2042 CrossRef CAS PubMed.
- R. J. Vandebriel, et al., Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats, Part. Fibre Toxicol., 2014, 11, 21 CrossRef PubMed.
- D. Westmeier, et al., Correction: Nanoparticle binding attenuates the pathobiology of gastric cancer-associated Helicobacter pylori, Nanoscale, 2020, 12(3), 2154–2155 RSC.
- M. D. Boudreau, et al., Differential Effects of Silver Nanoparticles and Silver Ions on Tissue Accumulation, Distribution, and Toxicity in the Sprague Dawley Rat Following Daily Oral Gavage Administration for 13 Weeks, Toxicol. Sci., 2016, 150(1), 131–160 CrossRef CAS PubMed.
- X. Ma, et al., Correction to Evaluation of Turning-Sized Gold Nanoparticles on Cellular Adhesion by Golgi Disruption in Vitro and in Vivo, Nano Lett., 2020, 20(1), 799 CrossRef CAS PubMed.
- Y. S. Kim, et al., Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats, Inhalation Toxicol., 2008, 20(6), 575–583 CrossRef CAS PubMed.
Footnote |
| † Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |