Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Renal tissue engineering for regenerative medicine using polymers and hydrogels
Syed Mohammad Daniel
Syed Mohamed
 a,
Gavin I.
Welsh
a,
Gavin I.
Welsh
 b and
Ipsita
Roy
b and
Ipsita
Roy
 *a
*a
aDepartment of Materials Science and Engineering, Faculty of Engineering, University of Sheffield, Sheffield S37HQ, UK. E-mail: i.roy@sheffield.ac.uk
bRenal Bristol, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol BS1 3NY, UK
First published on 23rd June 2023
Abstract
Chronic Kidney Disease (CKD) is a growing worldwide problem, leading to end-stage renal disease (ESRD). Current treatments for ESRD include haemodialysis and kidney transplantation, but both are deemed inadequate since haemodialysis does not address all other kidney functions, and there is a shortage of suitable donor organs for transplantation. Research in kidney tissue engineering has been initiated to take a regenerative medicine approach as a potential treatment alternative, either to develop effective cell therapy for reconstruction or engineer a functioning bioartificial kidney. Currently, renal tissue engineering encompasses various materials, mainly polymers and hydrogels, which have been chosen to recreate the sophisticated kidney architecture. It is essential to address the chemical and mechanical aspects of the materials to ensure they can support cell development to restore functionality and feasibility. This paper reviews the types of polymers and hydrogels that have been used in kidney tissue engineering applications, both natural and synthetic, focusing on the processing and formulation used in creating bioactive substrates and how these biomaterials affect the cell biology of the kidney cells used.
Introduction
About 10% of the world's population is affected by kidney diseases.1 It is considered a global burden by World Health Organisation, with 5–10 million deaths estimated annually due to this condition.2 Kidney failure or end-stage renal disease (ESRD) occurs when chronic kidney disease reaches an advanced state with the kidney having less than 15% of its usual efficiency both as a crucial excretory organ to filter the blood and in maintaining the homeostatic balance in humans. The most common causes that lead to kidney failure are diabetes and high blood pressure. ESRD is categorised into two major groups, acute kidney diseases and chronic kidney diseases.Acute kidney disease (AKD) or acute kidney injury (AKI) is defined as the rapid deterioration of kidney functionality, usually within hours, which involves structural damage and loss of functionality.3,4 These conditions are incurred abruptly, typically due to a physical blow during an accident, by a severe infection or sepsis,5 or ischemia; restriction of blood supply to carry oxygen to tissues,6 and kidney reperfusion that causes tissue injury.7 Besides that, autoimmune diseases may also contribute to this type of kidney failure, such as systemic lupus erythematosus, when autoimmune antibodies attack body tissues, and vasculitis, when the immune system attacks and causes detrition of blood vessels.8 Current diagnosis of AKI is based on the drop in glomerular filtration rate (GFR) value, and the elevation of serum creatinine (sCr) level, and urine output. These criteria classify AKI, as laid out in RIFLE staging, or risk-injury-failure-loss-ESRD, by assessing the GFR post-injury.3
Chronic kidney disease (CKD) happens when a kidney fails due to some detrimental effects on the vessels within the kidney, especially in the glomerulus. They mostly give rise to problems specific to the glomerular vasculature and the nephrotic tubular structure, such as tubulointerstitial inflammation, tubulointerstitial fibrosis, glomerulosclerosis, glomerulonephritis and glomerular hypertrophy. Glomerulonephritis is the inflammation of the glomerulus due to certain conditions which remove its ability to filter blood. Just like AKI, the effect can be assessed by the kidney's GFR. Besides GFR, albuminuria, the presence of albumin or protein in the urine also indicates a CKD problem.9 Common causes of CKD include high blood pressure when continuous strain damages small vessels within the kidney10 and diabetes, caused by metabolic changes due to hyperglycaemia.11 There are several other causes, including autoimmune disease such as lupus nephritis,12 a congenital condition such as polycystic kidney disease, a state where the kidney contains multiple fluid-filled cysts,13 neuropathy such as diabetic and obstructive nephropathy,14 and also urinary tract condition such as reflux nephropathy, a condition where kidney scarring happens due to urine backflow from the bladder towards the kidney,15 can cause CKD. These conditions damage the kidney, initially affecting filtration efficiency and, eventually, its overall functionality, both mechanically and physiologically.
This article reviews current treatment options for kidney diseases and explores the progress made in engineering kidney tissue. The current treatment for CKD and ESRD has been briefly described. Then, the need for kidney tissue engineering for regenerative medicine purposes is emphasised along with current strategies that have been used. The article continues with a detailed review of materials, specifically polymers and hydrogels, both synthetic and natural, used in exploring their compatibility with kidney cell culture. The content primarily focuses on using biomaterials in research efforts and strategies to develop functional kidney tissues by utilising these materials through fabrication and modification. This review will shed light on the advancements and challenges in creating functional kidney tissues for potential therapeutic interventions by examining these biomaterials, their properties, and their applications in kidney tissue engineering.
Current treatment for CKD and ESRD
Current treatment options for ESRD are inadequate as there is a lack of suitable donor organs for transplantation, and conventional haemodialysis acts merely as a filter without replacing the normal physiological, metabolic, endocrine and regulatory functions of the kidney.3,4 Hence, novel treatment approaches are urgently required. There is increasing interest worldwide in developing a bioartificial kidney using tissue engineering. This is a difficult problem since the kidney is a complex organ and consists of at least 26 types of cells operating in a single system.16–19Haemofiltration by dialysis
There are two well-known approaches to addressing CKD and ESRD. One approach is dialysis, an artificial way of undergoing haemofiltration that the diseased kidney lacks. This method was invented by a Dutch physician, Willem Johan Kolff, in the 1940s using a tank equipped with cellulose membrane and tubing that allows blood to flow out of the patient's body into the haemofiltration unit. The Kolff-Bringham dialyser uses the osmosis principle to diffuse the waste and excess fluid across the membrane into the dialysate. His dialyser prototype pioneered the modern dialysis machine.20 Peritoneal dialysis is another type of dialysis that relies on the abdominal lining, i.e. the patient's peritoneum to perform haemofiltration. A dialysate is introduced within the peritoneal cavity, allowing excess fluid and waste from the blood vessels to pass through the peritoneal membrane by diffusion.21 The dialysate is later drained and replaced with a fresh one.Dialysis is the main and much accessible treatment that has been used worldwide since kidney failure affects millions of people worldwide. According to the United States Renal Data System (USRDS) 2020 Annual Data Report, more than 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people in the United States and 2.6 million worldwide undergo dialysis per annum.22 Therefore, kidney failure is very common; and because of this, dialysis is also common and deemed as a conducive treatment. However, it does not improve the quality of life of a patient. They need to be attached to the dialyser for a standard four-hour session frequently, through a surgically made arteriovenous fistula, up to four times a week.
000 people in the United States and 2.6 million worldwide undergo dialysis per annum.22 Therefore, kidney failure is very common; and because of this, dialysis is also common and deemed as a conducive treatment. However, it does not improve the quality of life of a patient. They need to be attached to the dialyser for a standard four-hour session frequently, through a surgically made arteriovenous fistula, up to four times a week.
Dialysis is also very expensive and requires several types of drugs to balance some dialysis-induced conditions. One of the common drugs used is an erythropoiesis-stimulating agent to address anaemic episodes among diabetic patients and allow the formation of new blood cells.23 Hypertension is also common among the patients, and medication such as angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blockers (ARBs), beta-blockers and calcium channel blockers help to decrease complications such as heart conditions.24 Other supplements, help in alleviating deficiencies such as iron and vitamin D, while a phosphate binder reduces phosphate in the blood due to ineffective phosphate elimination by the impaired kidney.
Kidney transplantation
Kidney transplantation is another treatment for CKD and ESRD, mainly for patients suffering from glomerulonephritis, diabetic nephropathy and polycystic kidney disease.11 The procedure introduces a healthy kidney into a patient, surgically, to replace the diseased one. The first ever successful human kidney transplant was done by Dr Richard Lawler in Illinois, USA, on a lady with polycystic kidney disease.12 Through the procedure, a healthy and functional kidney is harvested from a living donor or a deceased donor and transplanted into the patient.Although this procedure is ideal in significantly improving the quality of life for the patient with kidney failure, the major hurdle is finding a suitable donor. In fortunate cases, the patient might have a donor among family members willing to donate one of the pairs and is physiologically compatible. Otherwise, the patient must be on an extraordinarily long waiting list to get an organ from a deceased donor. The challenge continues as the patient needs immunosuppressive agents such as calcineurin inhibitors, antiproliferative agents that suppress immune cells, and corticosteroids to reduce inflammation. Some of them might need to start a diabetic medication regime for new-onset diabetes after transplantation or NODAT, or even those with diabetes pre-transplantation.25 Given that some patients are not accustomed to a healthy lifestyle, they are prone to relapse and face the risk of failure of the transplanted kidney.
The available treatments for kidney failure, which are haemodialysis and kidney transplantation, provide an option to manage renal patients. While they have proven to prolong life expectancy, their effectiveness and feasibility may vary across individuals. Therefore, personalised approaches are necessary since the needs of each patient needs are different.
Kidney tissue engineering
Tissue engineering research provides the opportunity to mimic organs and potentially develop fully functional organs to replace diseased or damaged ones. A suitable material that serves as a biological substrate is vital to promote the required cell proliferation, maintenance, and maturation, to allow functional tissues to develop at the cellular level. There are three aspects of kidney functionality that need to be addressed in any model, specifically in kidney tissue engineering; haemofiltration (glomerular cells), reabsorption of water (tubule cells) and metabolic and endocrine activities (interstitial cells).18Organ shortage is the main reason of why transplantation is not usually an option in the event of organ failure, including the kidney. Tissue engineering strives to address this problem by developing an improved treatment option. Since tissue engineering is a type of personalised medicine, this approach would reduce the risk complication and be less heavily dependent on medication by considering patient's immunological and physiological need, case by case.26 Also, tissue engineering offers a step forward in developing an advanced diagnostic tool to detect disease in patients without major intervention, in the form of in vitro organ models. Again, the diagnosis can be tailored to a specific patient's condition and generate results for precise treatment regimes.
Kidney tissue engineering has been attracting major attention and in multiple ways, scientists are attempting to recreate a functional kidney. Since it is a relatively new area, it is important to understand (1) the cellular biology of kidney cells and potential materials that may support the growth of kidney tissue; (2) the selection of suitable biomaterials is crucial, especially with respect to biocompatibility, i.e. the lack of any adverse effects that hinder development of functional tissue; and (3) the fabrication of the biomaterial should be precise in order to facilitate tissue engineering in three-dimensional scaffolds to imitate the native structure of cell organisation in the kidney. Other methods, such as enhanced biocompatibility induced by material modification and creation of a drug release system, provide added value to the engineered scaffold.
The materials most frequently used in kidney tissue engineering are polymers and hydrogels. Polymers are known for their processability to replicate kidney features in terms of mechanical properties. In addition, polymers can be blended with bioactive components such as an extracellular matrix and growth factors that would in turn enhance the bioactivity of the polymer.27,28 Hydrogels, on the other hand, are widely used to imitate the extracellular matrix in vivo, as well as carriers for the cells, and are commonly used in cell therapy approaches. Furthermore, polymers and hydrogels are very robust and amenable to majority of the material processing techniques, for example, 3D printing and electrospinning for shape-tailored scaffolds, salt-leaching for introducing porosity in polymers,28 polymer emulsification method to control the size of cells encapsulation, and development of bioinks for spatially controlled cell culture using 3D printing of hydrogels.29
Brief overview of kidney tissue engineering: strategies and application
Several attempts at kidney regeneration have been undertaken using allotransplantation of kidney components with the goal of restoring the original functionality of the kidney. Current work is mainly based on small animal models, i.e., rats and mice. Even though the animal study may not be considered a perfect representative of human physiological makeup, especially by direct comparison to the human kidney's complexity, it serves as a preliminary tool in shedding light on certain biological mechanisms, in addition to gaining validation through proof-of-concept experiments. Some approaches include the implantation of embryonic nephros into the kidney, implantation of nephros beneath the renal capsule, in situ kidney regeneration, utilisation of stem cells and bioengineering of an artificial kidney.18Allograft of kidney tissue
Starting with the allograft approach, researchers introduced foetal renal tissue into a specified location, such as the renal capsule.30–33 However, this approach is only partially sustainable, with rejection in most cases. One set of studies focused on metanephrons, a type of renal tissue that is in the early structures involved in kidney development in an embryo. In murine models, allogenic grafts of embryonic metanephrons were transplanted into the anterior eye chamber and the renal capsule within the renal cortex, as a proof-of-concept study. These grafts showed high vascularity and the formation of new nephrons. Also, glomerular and tubular cells in the graft exhibited cytodifferentiation. However, graft rejection occurred after approximately 16 days.30 Another approach involved the allograft of adult and foetal renal tissue beneath the murine renal capsule. The adult renal tissue experienced rejection after 10 days, while the foetal renal tissue demonstrated growth, neovascularization, and limited signs of rejection after 10 days.31 In a different study, renal tissue derived from foetal midgestational tissue grafts were placed beneath the renal capsule. It was observed that the graft had a prolonged survival due to the lack of major histocompatibility complex or MHC, class I and II mRNA production. Furthermore, the transplantation of human foetal renal tissue revealed that the immune response leading to kidney rejection was dependent on the cell source, with foetal grafts exhibiting a favourable allogenic response for implantation.32,33On the other hand, an extraordinary attempt with a chimeric animal model approach involving kidneys from murine and avian sources observed the development of glomeruli and tubules, which extended to the medulla of the kidney after transplantation.34 In another study a similar approach was adopted, utilising gelatine microspheres as a cell carrier for rat kidney tubular cells in a cellular therapy approach. Some neovascularisations had occurred that may promote the development of healthy kidney structure.35
In summary, the tissue engineering approach involving the implantation of early-stage kidney tissue derived from an embryo was deemed feasible in repairing damage in the injured kidney. This tissue possess a greater capacity for proliferation and differentiation ability due to the availability of progenitors that can condition themselves in the new environment. This is crucial in developing fully functional and properly organised tissue. In contrast, the readily developed and differentiated adult kidney tissue may not properly adapt and thrive to give rise to a functional tissue; hence the chance of rejection is high. However, further optimisation still needs to be carried out since the goal is to enable essential functions of the kidney, especially haemofiltration and water reabsorption. The studies are summarised in Table 1.
| Cells/tissue involved | Source | Strategies | Results | Ref. |
|---|---|---|---|---|
| Metanephrons; nephron | Murine | Allogenic graft of embryonic metanephrons into the anterior eye chamber and renal capsule, within the renal cortex | Highly vascularised | 30 |
| New formation of nephrons | ||||
| Cytodifferentiation of glomerulus and tubular cells | ||||
| Graft rejection after 16 days | ||||
| Allograft of adult and foetal renal tissue beneath the renal capsule over the renal parenchyma | Adult renal tissue: rejection after 10 days | 31 | ||
| Foetal renal tissue: growth and neovascularisation after 10 days, little sign of rejection | ||||
| Midgestational renal graft beneath the renal capsule | Prolonged survival of the immature graft due to lack of major histocompatibility complex (MHC) class I and II mRNA production | 32 | ||
| Transplantation of human foetal renal tissue | Immune response of kidney rejection dependent on cell sourcing (foetal or adult) | 33 | ||
| Foetal graft has allogenic response favourable for implantation | ||||
| Murine and avian | Creating chimeric kidney by implanting avian and murine embryonic renal tissue to avian mesonephric mesoderm or cortex of the murine neonatal kidney | Quail: bilobed organ developed | 34 | |
| Implantation of nephrons into tunnels fashioned in the cortex which are eventually incorporated into the collecting system when the glomeruli were vascularised, formation of proximal tubules, extension of metanephric tubules | Mouse: postnatal transplanted metanephric tissue grew and developed glomeruli, proximal tubules and cords of cells extended to the medulla | |||
| Tubular cells | Rodent | Encapsulating kidney cells into cross-linked gelatine microspheres injected orthotopically and conducted in vivo assessment by histological evaluation | Observed non-excessive fibro cellular response and some interstitial inflammation and neovascularisation | 35 |
Development of bioartificial kidney
The main aim of developing a bioartificial kidney is to address shortages in artificial kidneys. Bioartificial kidneys work with the incorporation of renal cells within an engineered artificial construct, which would extend the device's functionality with the presence of metabolically active components. Since the kidney's primary function is to filter blood, hence, the focus is to create a bioartificial filtration barrier that can mimic in vivo haemofiltration mechanism. The tissue engineering strategy for this objective is to create a confluent monolayer of kidney cells on a support material. Then, the cell-scaffold hybrid will serve as a filtering membrane while sustaining cellular growth over time.The emphasis of the bioartificial kidney is the presence of living cells within the construct. Hence, their metabolic activities are the key focus in ensuring optimum performance and determining the feasibility of the artificial environment. In renal epithelium, for instance, metabolic activities such as ammoniagenesis; a way to excrete excess acids, production of calcitriol; responsible for the activation of vitamin D3, and cytokine response to endotoxin are the indication of its viability with proper functionality.36 Renal interstitial cells, on the other hand, produce a hormone called erythropoietin responsible for blood production.37
A limited number of bioartificial kidney devices have been developed with viable renal cells. Examples are the renal assist device (RAD) and bioartificial renal epithelial cells system (BRECS), which use proximal tubule cells and renal epithelial cells, respectively. These devices have been involved in pre-clinical trials in animals in the form of extracorporeal circuit devices. They have successfully led to blood filtration and restoration of metabolic components of blood (Table 2).
| Animal | Cells involved | Material involved | Strategy | Results | Ref. |
|---|---|---|---|---|---|
| Uremic dogs | Human and porcine proximal tubule cells | Polysulphone hollow fibres | Development of RAD containing human renal cells in an extracorporeal circuit | Increased excretion of ammonia, glutathione metabolism and 1,25-dihydroxyvitamin D3 | 17 and 38 |
| Anephric sheep | Renal epithelial cells | Porous carbon discs within carbonate housing | Usage of BRECS with continuous flow peritoneal dialysis circuit benefiting the peritoneal dialysis fluid to sustain the cells in the device | Retained neutrophil oxidative activities | 36 |
| Improved the immunological homeostasis and endocrinal needs in uremic condition |
Despite the favourable outcome, the cellular component of the RAD approach and BRECS only reinstate the essential metabolic activity of kidney cells. The involvement of a synthetic haemofiltration device equipped with a size-selective membrane is still essential to remove toxins from the blood. Hence, blood ultrafiltration still relies on artificial components within the system. Hence, the need to recreate a bioartificial filtration barrier to replace the synthetic unit is imperative in completing the endeavour towards developing a bioartificial kidney.
The development of a bioartificial filtration barrier mainly aims to re-establish the distinctive feature of the glomerulus in filtering blood. In addition, the presence of cells is allowing a myogenic response such as in the blood vessel, assumed to be from endothelial cells for renal autoregulation to happen in order to regulate the glomerular blood flow.39 The engineered construct will provide more physiological relevance to the kidney tissue engineering model.40
Polymers in kidney tissue engineering
Polymers have emerged as potential materials in scaffold development in tissue engineering, including kidney tissue engineering. Their versatile properties allow easy fabrication, which is important to mimic the extracellular matrix in native tissue in terms of integrity and bioactivity. They are well-known to play an important role in creating a suitable substrate and providing structural framework to allow cell proliferation and differentiation. In kidney tissue engineering, the polymeric material should ideally possess mechanical properties that allow withstanding the shear force from blood flow and porosity to allow gaseous exchange and nutrient transport in addition to being biocompatible. Polymers can be synthetic or natural, and both type exhibit benefits towards perfecting the design of the kidney tissue engineering scaffold. Synthetic polymers are usually known for structural integrity and tailorable degradability, meanwhile natural polymers are known to be highly biocompatible for the application.Synthetic polymers
In general, synthetic polymers exhibit tailorability and customisable properties to fit a specific application. For instance, polylactic acid and polyglycolic acid can be synthesised so that the product would possess the specific chemical composition needed. These can even be co-synthesised to produce polylactic-co-glycolic acid in order to further broaden the range of mechanical properties, and controlled degradation.41,42PS has significant potential in developing bioartificial kidneys to perform haemodialysis due to its rigidity.45–47 Scientists have created a more functional material, improving its physical attributes, such as hydrophilicity, by blending with reactive components and surface modification.48 A clinical study of a bioartificial kidney equipped with a PS membrane has been created using Lewis lung cancer-porcine kidney 1 or LLC-PK1 and Madin–Darby canine kidney (MDCK) cells (Fig. 1). The cells were seeded onto PS coated with extracellular matrices (ECM) such as collagen type I, laminin and pronectin-F and then assessed for monolayer formation and functionality.49 The device managed to decrease the amount of urea, uric acid, and creatinine by up to 50% and β2-microglobulin under 20 mg L−1 in a human patient.50
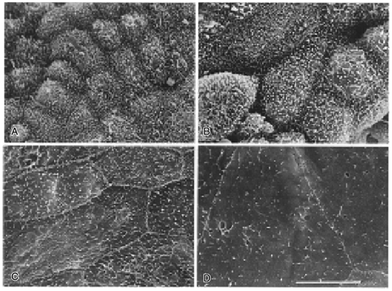 | ||
| Fig. 1 Electron micrographs of MDCK cells, renal tubular cells on PS (A) one week, (B) two weeks, (C) three weeks, and (D) four weeks. The scale bar size is unknown. Republished from Saito, 2004 with permission.50 | ||
Ongoing kidney tissue engineering studies that utilise PS biocompatibility have led to the development of superior bioartificial devices for haemodialysis. One study focused on attaching two types of renal cells, human kidney 2 (HK-2) proximal tubule cells and Madin-Darby canine kidney (MDCK) epithelial cells, to fabricate PS hollow tubes. The tube fibres were prepared by extruding polymer-in-solvent solution through double injection nozzles with different diameters to create different tube curvatures. Assessment of the water flux showed significant ultrafiltration properties, between 190–256 L m−2 h−1, with a high bovine serum albumin rejection percentage, i.e. above 70%. All cells managed to achieve confluent growth on the materials. Ultimately, higher curvature or lesser diameter of the hollow tubes promoted cell functionality, allegedly due to mechanical stress akin to natural minuscule tubular kidney architecture.46
Meanwhile, another PS fibre membrane fabrication was investigated for its enhanced biocompatibility and ability to remove uremic toxins. Before cell culture, the membrane was coated either with a single coat of D-α-tocopheryl polyethylene glycol 1000 succinate; or a double coating of L-3,4-dihydroxyphenylalanine (L-DOPA) and human collagen type IV to improve hydrophilicity and biocompatibility towards human blood. Human embryonic kidney cells 293 (HEK 293) were used and observed to form a confluent monolayer on the coated membrane, indicating improved biocompatibility. As tested in the study, the membrane also managed to effectively remove uremic toxins, such as urea, creatinine, and phosphorus, to a significantly greater extent than the commercial PS membrane. This suggests this fibre membrane has excellent potential for developing bioartificial kidneys as a ‘living’ haemofilter.49
PS, specifically PES-50, was coated with L-DOPA and human collagen type IV before cell culture involving a conditionally immortalised proximal tubule epithelial cell (ciPTEC) line. The optimised coating promoted water permeability and cell monolayer formation, as well as retained proteins such as bovine serum albumin and immunoglobulin G.51 A similar investigation involving different fabricated PS membrane surface designs were carried out. PS was blended with polyvinyl pyrrolidinone K90 or PVP and made porous by a phase separating micro-moulding technique,52 using a specified silicon mould design produced by photolithography. CiPTEC were also used in this study, cultured onto them, and observed. A confluent monolayer was easily formed by the cells on the membrane with small features and wider gaps without any coating compared to the larger ones. Different topographical arrangements of PS were concluded to have the ability to influence cell orientation and morphology, defined by the size and gaps of micro-features that are distributed on the membrane.53
The work by Basu et al.57 assessed and compared the interaction of different kinds of polymer with kidney cells. At first, the use of PCL was not promising; direct implantation of PCL beads into healthy adult Lewis rat kidneys caused an inflammatory reaction in the first week, which continued leading to dilation and hydronephrosis after 4 weeks. Direct seeding of sunitinib-resistant renal carcinoma, or SRRC cells onto PCL also showed poor adherence after one day of culture.57 Despite this, modification of PCL has resulted in it becoming a suitable kidney tissue engineering material. Physical modification of PCL has been carried out by electrospinning, a method that extrudes polymer fibres with an electrical charge from melted polymer or polymer solution. Work by Burton et al.,58 for instance, produced different types of PCL-electrospun fibres, random, aligned, and cryogenic, for kidney tissue engineering purposes. The polymer fibre scaffolds were plasma-treated to introduce hydrophilicity before cell culture. The fibres supported the growth of human kidney primary epithelial (RC-124) cells regardless of fibre orientation. However, the growth was improved with larger diameters, presumably due to a higher degree of porosity that promotes cell incorporation.58 Another group incorporated laminin, a component of the extracellular membrane, into the electrospun PCL to form a hybrid scaffold to enhance the bioactive properties. This scaffold supported RC124 kidney cell growth (Fig. 3). The cells were metabolically active across 21 days of culturing and showed an increase in E-cadherin expression, a component responsible for cell junction formation.59
 | ||
| Fig. 3 RC-124 cells, a type of renal epithelial cells on PCL electrospun fibres scaffold. (a) Neat PCL, (b) PCL prepared by blending with laminin, and (c) PCL prepared by emulsification with laminin. Adapted from Baskapan & Callanan, 2021.59 Copyright © the authors. | ||
Another exciting approach is a way to use a PCL-based material with polyethylene glycol, or PEG, as a coating material. E-caprolactone was co-synthesised with PEG to produce PCL-PEG-PCL, which is more hydrophilic due to the PEG component.60 Neat PCL was initially prepared by Fused Deposition Modelling (FDM) to create a 3D scaffold with a criss-cross design, later spray-coated with the triblock copolymer. The coated PCL was reported to promote three times higher cell growth of embryonic kidney cells than non-coated PCL, with no cytotoxicity response.61
Other PCL modification approaches to promote bio-responsive properties have been undertaken, such as surface modification with arginyl-glycyl-aspartic acid or an RGD peptide motif to enhance the cell attachment.62,63 However, this technique is yet to be explored with kidney cells as a novel functional material development.
A cytocompatibility test was carried out using primary kidney cells derived from rats on electrospun PLA fibres of different diameters. The scaffold fabrication produced fibres of different diameters; 0.88 ± 0.16 μm for small fibres, 2.46 ± 0.43 μm for medium fibres, and 3.30 ± 0.17 μm for large fibres. Furthermore, the introduction of a cryogenic condition when collecting the fibres also yielded a slightly larger fibre diameter at 3.71 ± 0.36 μm. Interestingly, this fibre was the best at supporting cell proliferation by having the highest DNA content after three and seven days of culture, which may be due to its higher porosity. Overall, protein assays confirmed the viability of four types of kidney cells on PLA, namely the proximal tubules, collecting ducts, podocytes, and glomerular endothelial cells (Fig. 4).67
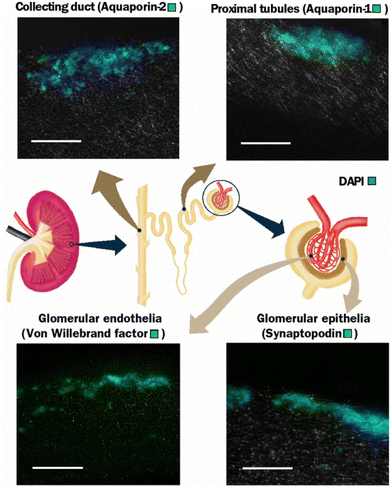 | ||
| Fig. 4 Different types of renal cells culture showing expression of respective protein expression by immunohistochemical staining using a PLA scaffold at 7 days. Scale bar is 100 μm. Adapted from Burton & Callanan, 2018.67 | ||
Another electrospinning technique was adopted to fabricate PLA for a similar application. This work addressed the hydrophobicity of PLA by adopting coaxial electrospinning with polyvinyl alcohol or PVA, which drastically increased the wettability by more than four times compared to neat PLA. A cell compatibility test was done using HEK 293 cells; however, it showed that neat PLA still performed as the best scaffold supporting up to 75% cell viability, compared to fabricated PLA fibres supporting only 35–40% cell viability. Even though fabricated PLA fibres do not demonstrate as high cell viability as neat PLA, SEM imaging confirmed that they do support the HEK 293 cell attachment.68 It is hypothesised that neat electrospun PLA produces more porous fibres that possibly influence the attachment of cells, resulting in high viability.
In a study of the effect of a 3D scaffold on glomerular cells, PGA was fabricated with fibrin gel to improve its bioactive properties upon culture. Two types of conditionally immortalized human glomerular cells were used, podocytes and glomerular endothelial cells. The unique scaffold was seeded with either mono- and co-cultures, with the co-cultures exhibiting an interesting self-assembly behaviour besides displaying good proliferation and cell adhesion patterns. Importantly, expression of collagen IV, a key glomerular basement membrane (GBM), was confirmed, showing the potential of the scaffold in developing a kidney filtration barrier.40
An assessment of PLGA as a polymer scaffold for kidney tissue engineering was done by Basu et al., who compared it with PCL.57 A week after injection of neat PLGA particles into the medulla and cortex of the kidney of living Lewis rats, no necrosis, embolism, or infarction was observed; however, there was chronic inflammation and formation of granulomatous cells (giant cells) around PLGA at the medulla. Meanwhile, implantation of PLGA beads showed induced embolism and acute infraction at the cortex, as well as minimal fibrosis, chronic inflammation and also formation of granulomatous cells. Despite that, the implantation of a porous PLGA scaffold equipped with magnesium hydroxide as an anti-inflammatory agent and porcine renal extracellular matrix into a nephrectomised mouse kidney demonstrated the regeneration of the glomerulus, interestingly, later restoring the kidney function for the mouse.73
Several silicon-based materials have been fabricated into a nanopore membrane, adapting sophisticated step-by-step wafering and coating techniques. The fabrication involved a set of silicon-based components, namely single-crystal silicon, polycrystalline silicon, silicon dioxide, and silicon nitride. The nanopore membrane design was developed with different pore sizes ranging from 10 nm to 500 nm as a haemofilter. Initially, human cortical tubular epithelial cells (HCTC) were grown to form a monolayer, separately with each component for cytocompatibility and showed consistent and favourable growth. Meanwhile, cell behaviour on the fabricated silicon nanopore membranes allowed cell differentiation with cilia and tight junction formations.76
A strategy using a ribbed design membrane involving silicon and polysilicon components as a potential haemofilter in a bioartificial kidney has been developed.77 Surface modification of silicon has also been investigated to enhance wettability and promote cell adhesion, such as by hydrosilylation.78 Although, to date, there is a lack of reported work using silicon-based kidney tissue engineering, this material exhibits the potential to be used to successfully develop a bioartificial haemofiltration membrane, which might also be a cell-seeded ‘living’ membrane on a smaller scale for transplantable devices.
Natural polymers and biopolymers
There is little reported so far on utilising cellulose as a material to be used for dialysis in order to address kidney failure. MacLeod et al. compared cellulose membranes from regenerated cellulose sourced from cotton with polysulphone and showed cellulose exhibits less biocompatibility and more immune response than synthetic membranes. The authors, however, concluded that the membrane replacement did not contribute to adversity for patients undergoing haemodialysis.71,72 In other reported studies, further fabrication of cellulose into cellulose triacetate73 or cellulose diacetate74 reduced platelet activation, making it closer to the commercial dialyser membrane properties in terms of biocompatibility for a more feasible product.
In kidney tissue engineering, the involvement of cellulose-based hydrogels has been investigated as a potential scaffold for cell growth. UPM Biomedicals has developed a product called GrowDex® nanofibrillar cellulose (NFC) hydrogel for kidney organoid growth, using primary embryonic metanephric mesenchyme of murine source (Fig. 5). The material successfully provides a 3D culture of the kidney cells and demonstrates a chemically induced nephrogenesis of the organoid.75 This product is deemed a potential drug testing, disease model and a kidney development and regeneration study model.
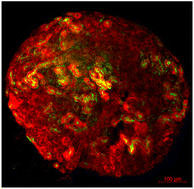 | ||
| Fig. 5 Renal organoid cultured in GrowDex®. Organoids were stained with Pax2 (kidney tubules, red) and Lotus Tetragonolobus Lectin (LTL, proximal tubules, green). | ||
Meanwhile, a unique approach to develop a cellulose-based scaffold for kidney tubule tissue engineering has been carried out using spinach and chive as base materials. The wet market-bought vegetables are decellularised using a 5% v/v sodium dodecyl sulfate (SDS) solution for 7 days. The scaffold is then coated with L-3,4-dihydroxyphenylalanine (L-DOPA) to introduce a bioactive surface to allow cell attachment. Conditionally immortalised proximal tubule epithelial cells (ciPTEC) were used to seed the scaffold. It is concluded that spinach and chives cellulose matrix are not favourable for fostering transepithelial solute exchange due to the micro-anatomical structure of the scaffold providing a lack of permeability.80
Further work focused on developing a functional material with cellulose, specifically using bacterial cellulose for a tissue engineering application. The cellulose was produced by fermentation of Gluconacetobacter saccharivorans LMG 158, in parallel fed with D-glucose and cultured with the presence of carboxymethyl cellulose sodium salt and hydroxyapatite for in situ incorporation. The cytocompatibility of the composite material was tested using HEK 293 cells, showing high cellular viability up to 97.2%.81 However, further strategies are needed to promote cell adherence since despite the high viability of the matrix, the cells were not attaching to the BC surfaces.
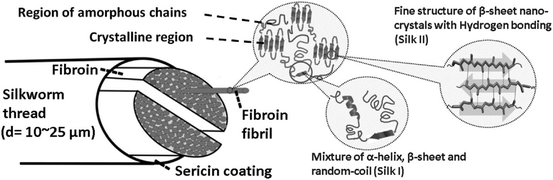 | ||
| Fig. 6 Schematic diagram of silk. Reproduced with permission from Volkov et al., 2015.148 | ||
Organoids are being increasingly used to replicate much of the complexity of an organ, and silk has proven to be a great substrate for these applications due to its high cytocompatibility as well as significant cell adhesion property.88 Gupta et al. successfully developed kidney organoids induced from pluripotent stem cells with a silk scaffold through spin seeding, which supported differentiation into epithelial cells from kidney progenitor cells with nephron markers (Fig. 7).89 Engraftment of the organoid epithelial cells under the renal capsule showed vascularisation and induced mesenchymal cell proliferation within the scaffold. Despite this model lacking the cellular organisation akin to renal tissue, further fabrication plans are possible, as demonstrated by Szymkowiak et al., who developed an aligned silk sponge by directional freezing to imitate the kidney tubule structure. This scaffold was seeded with adult proximal tubule cells and cultured in a perfused reactor, which was proven to induce cell polarity.90 Upregulation of key proximal tubule markers, especially SLC9A3, a sodium-hydrogen exchanger protein, was observed in the perfused condition but not in the static, indicating that the fabricated silk scaffold is necessary for maturation. Hence, this method opens the potential for a bioartificial renal assist device with a close-to-real kidney component.
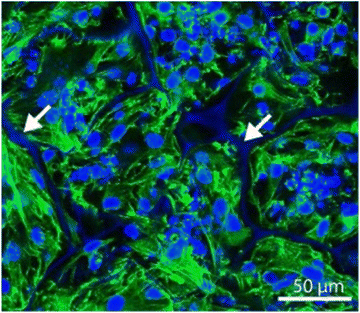 | ||
| Fig. 7 Kidney progenitor cells seeded on a silk scaffold, stained with Phalloidin 488 (green) and DAPI (blue), showing cells were packed into the scaffold. Reproduced with permission from Gupta et al. 2019.89 | ||
For disease modelling that addresses the morphogenesis of kidney epithelial cells, silk-based scaffolds were developed to compare healthy and diseased kidneys, focusing on autosomal dominant polycystic kidney disease. Subramanian et al. used murine kidney epithelial and fibroblast cells, which were co-cultured in a collagen-Matrigel matrix to promote morphogenesis before being infused into a porous three-dimensional cylindrical silk scaffold. The cell-scaffold system was later introduced into a perfused bioreactor setup and showed better structural development than a static culture. This strategy produced a sustainable tissue model with stability for up to six weeks for both healthy and diseased cells, given the low degradation property of silk, and most importantly, allowed for tissue morphogenesis that much better mimicked what is seen in vivo.91 Similar work from the same research group used the 3D printing technique to generate a porous silk scaffold in which normal or polycystin-1 silenced murine inner medullary collecting duct cells were mixed with a collagen-Matrigel matrix. The use of these scaffolds showed that in the silenced Pkd1 cells, there are autocrine signalling loops which lead to unusual matrix deposition and changes in the integrin-β1 protein subunit, leading to a higher rate of cystogenesis in the tissue.92
Silk has also been processed into fibres by electrospinning to serve as a tissue engineering scaffold considered biomimetic. Work performed by Mou et al. utilised podocytes derived from induced pluripotent stem cells and demonstrated maturation of the cells on the laminin-functionalised silk sheet for the first time with the expression of podocyte-specific markers such as podocin and nephrin. The cells were also sustained for up to two weeks.93
Hence, these results point to the utility of silk in kidney tissue engineering applications as a highly potential biomaterial.
Hydrogels in kidney tissue engineering
Bio-based hydrogels
Natural polymer-based hydrogels that have been considered suitable candidates, especially for kidney tissue engineering, include gelatin and collagen, known for manipulating the extracellular matrix protein composition.28 These materials are inherently biocompatible and non-toxic without significantly triggering immune responses within human physiology, which makes remodelling convenient. Since natural hydrogels are bio-based, they are easily functionalised to improve biocompatibility even if they are not bioactive. Also, the water retention capacity generally helps nurture cellular sustainability within a 3D structure. In kidney tissue engineering, renal researchers recognise the importance of renal cell growth, and strategies were developed to emulate an ideal cellular matrix in promoting bioactivity and cell signalling to mimic native environment.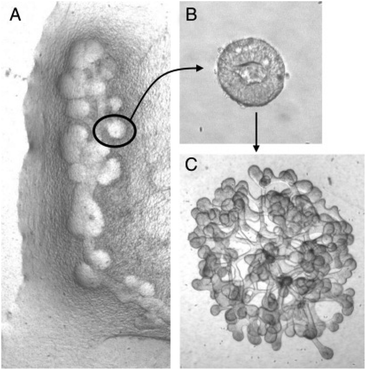 | ||
| Fig. 8 Figures of (A) budding of Wolffian duct, (B) isolated single bud cultured in 3D ECM matrix and induced to branch, and (C) branched organoid. Reproduced from Rosines et al.96 Copyright © 2007 by The National Academy of Sciences of the USA. | ||
Several ECM experiments have been carried out as a kidney regenerative medicine approach.94 One of the techniques is recolonising decellularised kidneys with kidney cells. The decellularisation is usually done using surfactants, normally sodium dodecyl sulphate (SDS) and Triton X-100, to wash out all cellular components leaving out only the ECM. Several types of cells have been used to recolonise these structures, such as primary renal cells,97,98 induced pluripotent stem cells,99–102 embryonic stem cells,103–105 and tubular cells106 showing positive outcomes in both supporting cell attachment and growth, as well as allowing urine production.
Kidney ECM also has been further processed and fabricated, demonstrating the processability and versatility of this biomaterial in tissue engineering applications. It has been lyophilised and cryomilled (Fig. 9), allowing composition tailoring to make up a scaffold as a hydrogel.107–111 Blending has been adopted as well, as reported by Lih et al., who incorporated kidney-derived ECM within PLGA 3D scaffold with magnesium hydroxide to address the acidification and inflammation response.73,112 Furthermore, ECM has been successfully electrospun to mimic the filtration barrier using PCL as the base material. The ECM promoted the formation of a tight junction,113 and brush-border microvilli and cell polarisation were also observed.114 Kidney ECM has also been made into “tissue paper” that can be cut, rolled, folded and sutured, which has been proven to be very porous at 85.5 ± 1.8%, and 2.4 ± 0.8 MPa of Young's modulus.115 Meanwhile, Matrigel, a type of ECM derived from Englebreth-Holm Swarm mice tumours consists mainly of laminin and collagen type IV, has also been used as a 3D matrix culture environment. Matrigel has the typical composition of glomerular and tubular basement membranes. In an attempt to design whole kidney tissue engineering for implantation applications, Matrigel was shown to be one of the best materials to support the branching of an isolated cellular bud derived from a rat mesonephric duct (Wolffian duct).96
 | ||
| Fig. 9 Step-by-step process obtaining ECM from kidney, (1) kidney collected, (2) cut into small pieces, (3) decellularised in SDS and Triton X-100, (4) lyophilised and ground into powder, and (5) rehydrated and solubilised. Adapted with permission from Magno et al., 2017.108 | ||
Another unique approach using ECM in kidney tissue engineering is the development of bioinks. One group developed a photo-cross-linkable ECM by introducing methacrylic components along the ECM fibres. Using a heterogeneous human primary kidney cell mixture, the bioink was formulated with thermosensitive gelatin, hyaluronic acid to promote uniform dispersion, and glycerol to assist the ink extrusion.116 Another bioink approach blended the ECM with sodium alginate to assist cell encapsulation which was later crosslinked by calcium chloride; in this case, using human proximal tubular cells, stem cells and human umbilical vein endothelial cells (HUVEC).117
Generally, ECM, especially kidney-derived, has been shown to support kidney cell growth and proliferation with good adhesion properties. For attempts to create an ideal kidney replacement method through a tissue engineering approach, ECM is deemed as an essential component, and several approaches rely on in situ excretion by the renal cells themselves to better mimic the ECM observed in vivo.
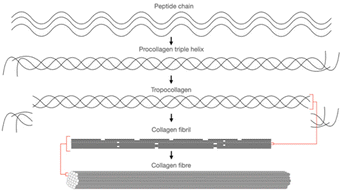 | ||
| Fig. 10 The building block of collagen from peptide chain to collagen fibre. Adapted from Kruger et al., 2013.121 | ||
In terms of cell culture, collagen type I extracted from the scales of Egyption Nile Tilapia, Oreochromis niloticas, has demonstrated cytocompatibility with a baby hamster kidney (BHK-21) cell line, with no toxicity effects observed, even across different collagen concentrations (Fig. 11).119 In another study, collagen type I extracted from the swim bladder of grass carp has been investigated for renal tissue engineering application; initially using protein functionalised with methacrylic anhydride to allow crosslinking for structural stability and blended with chondroitin sulphate as an antifibrotic component. This biomimetic hydrogel has been shown to heal nephrectomised rat kidneys by promoting kidney cell growth, regenerating damaged tubular structures and restoring cellular metabolic function.120
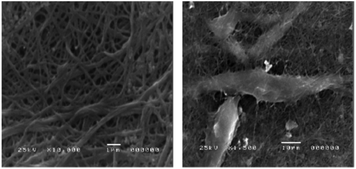 | ||
| Fig. 11 Electron micrograph of collagen from Egyptian Nile Tilapia, neat collagen (left) and collagen cultured with BHK-21 (right) showing good attachment. Reused with permission from El-Rashidy et al., 2015.119 | ||
Rehydrated collagen vitrigel (collagen type I), has been prepared by vitrification, a method of drying to form a glass-like material, and has been shown to support the co-culture growth of glomerular epithelial cells with renal mesangial cells, promoting the polarisation of cells observed in in vivo glomeruli.122 Meanwhile, the collagen-Matrigel matrix has been demonstrated as a scaffold that fostered the self-assembly of tubular and glomerular cells, with tube- and tuft-like architectures, respectively.123
In a scaffold engineering approach, collagen was used to create an in vitro biomimetic branched vasculature containing kidney scaffold. Laboratory-grade collagen type I was used to coat the PCL cast perfused in a rat kidney as mould. The PCL has been washed away with acetone, leaving a hollow collagen scaffold colonised with MS-1 endothelial cells to enhance vascularisation. The 3D construct can be perfused, endothelialised and vascularised (Fig. 12).124
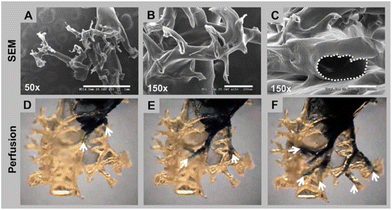 | ||
| Fig. 12 Branching and hollow structure of collagen vascular scaffold. The top row (A–C) shows the branching through electron microscopy, and the bottom row (D–F) shows the perfusion of trypan blue dye for the structure continuity and interconnection. Reproduced with permission from Huling et al., 2016.124 | ||
Given its bioactive property and as a naturally occurring component within almost all cellular environments, collagen is a great candidate to bring renal research forward. Whether developing a practical cell therapy approach or an ideal scaffold in tissue engineering, it provides a highly suitable tool for renal regenerative medicine.
For instance, a human renal progenitor cell was encapsulated in a gelatin-based hydrogel equipped with hyaluronic acid to address immunoglobulin A (IgA) nephropathy in a renal cell therapy study. The hydrogel was injected under the renal cortex of high serum IgA mice, also known as ddY or HIGA mice, a mouse strain that develops spontaneous IgA nephropathy, to enable treatment by a cell therapy approach. Injected mice were seen to have a normal appearance of the kidney with a decreased expression of pro-inflammatory and pro-fibrotic components, increased expression of anti-inflammatory genes, and much reduced IgA deposition.126 In another study, murine pluripotent embryonic stem cells were packed into a gelatin microcryogel as a cell carrier to regenerate kidneys damaged using the 5/6 nephrectomy model of chronic kidney diseases. The cell-hydrogel was wrapped with the incised kidney by the omental flap. In the treated animals, plasma creatinine levels decreased by 30–40% and plasma urea nitrogen by 20–26% after 12 weeks, and there was a marked reduction in glomerulosclerosis and tubular injury.127 Similar work from the same group utilising mesenchymal stem cells further demonstrated that this method ameliorated fibrosis and promoted antitubular inflammation suppressing CKD progression.128
In addressing the sensitivity of renal cells towards the mechanical properties of the material, work studying podocyte behaviour was conducted using gelatin as a culture substrate. The gelatin was enzymatically crosslinked by gelatin transglutaminase to link glutamine and lysine groups, producing a biomimetic matrix akin to a healthy glomerulus, with Young's modulus between 2–5 kPA. Interestingly, the podocytes expressed genes and proteins that reflect their specificity, differentiation, and functionality, in contrast to those cultured in a soft and stiff hydrogel.129 Gelatin was also fabricated into microspheres, crosslinked by a carbodiimide-based crosslinker solution to tune the mechanical property, in this case, to control its biodegradability. Instead of encapsulation, rat kidney cells were cultured and injected into rat kidney parenchyma. Beads with a lower degree of crosslinking were more susceptible to degradation, producing good cell performance without inducing fibrosis.35
Besides capsules and microspheres, the processibility of gelatin has enabled it to be electrospun to construct nanofibrous scaffolds. The initial hydrogel solution was formulated with an array of gelatin and acetic acid concentration ratios, in which the presence of acetic acid was to assist dissolution that leads to a tuneable electrospinning solution viscosity. A cytocompatibility test with human endothelial kidney cells, HEK 293 cells, demonstrated that electrospun gelatine scaffold with 25% acetic acid has the highest cell viability up to 90%, which was suggested to be due to the smallest amount of acid traces available, post-processing.130
In a sophisticated fabrication of a perfusable scaffold on-a-chip, a 7.5% w/v gelatin-fibrinogen matrix was housed in a microchannel formed by printing Pluronic F127 as fugitive ink. After flushing the fugitive ink, the channel was conditioned with media before being seeded by perfusion with renal proximal tubular cells. They maintained the culture for over two months, with clear epithelial morphology and functionality comparable to those in 2D structure (Fig. 13).131
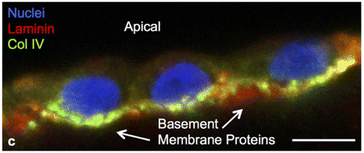 | ||
| Fig. 13 Podocytes expressing key basement membrane components namely laminin and collagen IV, at six weeks on a perfusable channel wall made with a gelatin-fibrinogen. The scale bar is 10 μm. Reused from Homan et al., 2016.131 | ||
In terms of developing bioink, gelatin is one of the most suitable cell carriers with an ideal melting temperature that could support kidney cells.116 A bioprinting approach utilising human endothelial kidney cells, HEK293FT, has been carried out and optimised to maintain the viability of the cells by more than 90% within the hydrogel matrix. The approach has formulated 10% of gelatin as the main component within the bioink, along with 1% alginate and 2% fibrinogen, supporting 3D cell growth into spheroids.132
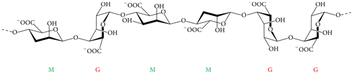 | ||
| Fig. 14 Alginate general molecular structure consists of β-D-mannuronic acid (M) and the C5 epimer α-L-guluronic acid (G), all linked by glycosidic bonds. Adapted from Szekalska et al., 2016.135 | ||
A cell therapy experiment was carried out in vivo and in vitro, using a formulation of decellularised porcine kidney extracellular matrix with alginate hydrogel crosslinked by calcium chloride solution. In vitro cytocompatibility study utilised rat renal progenitor cells, showing that a composition of 2% of alginate was optimal for supporting cell proliferation over 7 days of culture (Fig. 15). In vivo injection of the progenitor cell encapsulated hydrogel stimulated early-stage healing by accumulating M1 and M2 macrophages, along with hydrogel degradation over 21 days.136 A similar strategy encapsulated mesenchymal stem cells into alginate microspheres coated with poly-L-lysine hydrogel construct as a graft,137 which was intended for implantation as a cell-laden scaffold into an impaired kidney. The microspheres were shown to be stationary over 25 days, with no inflammation and fibrosis, and without significant change in renal function in terms of concentration of creatinine and urea in plasma, compared to sham rats as control.138
 | ||
| Fig. 15 Cell viability test of renal progenitor cells across different ECM/alginate blend compositions, showing that 2% of alginate has the best performance over seven days of culture in vitro where (a) CKK-8 assay and (b) confocal microscopy. Reproduced with permission from Chu et al., 2022.136 | ||
Alginate is also seen as a potential material to enable the engraftment of cells to a damaged kidney through advanced processing. A thiolene functionalisation technique was adapted to design an alginate-based hydrogel that is photo-crosslinkable. The soft hydrogel is an in vitro matrix to culture kidney organoids pre-implantation. It was prepared using norbornene functionalised alginate, mixed with PEG and lithium phenyl (2,4,6-trimethyl benzoyl) phosphinate (LAP) photoinitiator, and was shown to suppress the abnormal collagen type I α1 and α-smooth muscle actin (αSMA) production observed during fibrotic instances, nurturing proper organoid maturation in vitro.139 Another approach designed a biomimetic matrix for organoid culture utilising oxidised alginate (alginate with the C2 and C3 bond cleaved within the hexose ring, forming two aldehyde group).140 The structurally dynamic alginate produced benefited in terms of promoting most kidney cellular segments development, tubule polarisation and cilia formation. The stress-relaxing hydrogel as well eliminated early marker of renal fibrosis.141
Alginate was also used as a bioink in an extended application for encapsulating renal cells for 3D tissue engineering. An in vitro kidney model was developed via bioprinting, utilising primary murine tubular epithelial cells in combination with HUVEC cells within a concentric tubular design, spatially separating the two cells in a tubular structure using a core–shell printhead. The viability of cells over seven days of culture was not promising for both commercially obtained AG-10™ Matrix alginate and alginate from brown algae.142 It was assumed that further effort is required to make a more bioactive alginate bioink formulation. For bioengineering applications, alginate has mechanical versatility and is easily modified to improve its biocompatibility. Improving biocompatibility, especially for in vitro matrix design for renal culture, is crucial for renal tissue modelling or implantable organoid culture.
Synthetic hydrogels
Synthetic hydrogels are defined as a type of material that is chemically synthesised, which enables it to swell and retain water, as well as form a matrix. Typically, the building block of synthetic hydrogels is synthetic polymers. Polyethylene glycol, polyethylene oxide, polyvinyl alcohol, poly(2-hydroxyethyl methacrylate) polyacrylic acid, and poly(propylene fumarate-co-ethylene glycol) are some examples of synthetic, biocompatible, and gel-forming polymers suitable for tissue engineering applications.143 To date, there are limited reports on the use of synthetic hydrogels in kidney tissue engineering. Since one of the crucial aspects to enable cell growth is for any material to be bioactive, most hydrogels selected so far for this purpose have been naturally-derived.In the work carried out by Astashkina et al., a 3D kidney model was developed from a proximal tubule extracted from a murine kidney using a synthetic hydrogel-based matrix. Formulation of 7.5% polyethylene glycol dimethacrylate (PEGDA) and 1.5% thiol-modified carboxymethylated hyaluronic acid (CMHA-S) to introduce a bioactive environment, made up of the hydrogel to enable a 3D culture of the cells. The organoid formed is claimed to maintain cellular activity observed in vivo and was stable for up to six weeks, which is relevant for a drug screening model focusing on nephrotoxic effects.144,145 Clerkin et al. developed an organic-based synthetic hydrogel, gelatin methacryloyl, widely known as GelMA, to grow iPSC-derived kidney organoids. It has been shown to nurture the development of both distal and proximal tubular structures as well as the glomerulus, with gene expression analysis showing upregulation of nephron-related genes, including PAX8, NPHS2, NPHS1, SLC3A1 and AQP1.146
Synthetic hydrogels are the future of tissue engineering, with the possibilities of tailoring an ideal cell culture matrix. However, their bioactive properties still need to be properly addressed. Generally, cells in microenvironment need biochemical cues and signals that promote proliferation and differentiation in order to be fully functional. Kidney cells for instance respond well with presence of bioactive materials such as collagen118–120 and laminin,59 both of which are integral in healthy kidney tissues. Other types of biomolecules that can be considered include RGD-peptides to improve cells attachment onto the substrate,62 and growth factors such as vascular endothelial growth factor, or VEGF, especially for glomerular endothelial cells in order to promote vascularisation.147 Kidney extracellular matrix is one of the biomaterials that proven to nurture kidney cells, given all of its composition allow kidney cells to stay viable to become fully functional.
Hence, synthetic hydrogels are yet to be explored for their potential in kidney tissue engineering. The strategy for the enhancement of their biocompatibility and bioactivity by the incorporation of certain compounds in order to enable cell–matrix interaction and ultimately promote a better design for kidney regenerative application (Table 3).
| Material | Modification | Cell line | Results | Ref. |
|---|---|---|---|---|
| Synthetic polymers | ||||
| Polysulphone (PS) | Coated with extracellular matrices (ECM) such as collagen type I, laminin and pronectin-F | Lewis lung cancer-porcine kidney 1 or LLC-PK1 and Madin-Darby canine kidney (MDCK) cells | Resulted in a decreased amount of urea, uric acid, and creatinine by up to 50% and β2-microglobulin under 20 mg L−1 in a human patient | 49 and 50 |
| Creating hollow tubes by extruding polymer-in-solvent solution through double injection nozzles with different diameters to create different tube curvatures | Human kidney 2 (HK-2) proximal tubule cells and Madin-Darby canine kidney (MDCK) epithelial cells | Significant ultrafiltration properties were achieved, between 190–256 L m−2 h−1, with a high bovine serum albumin rejection percentage, i.e. above 70%; higher curvature or lesser diameter of the hollow tubes promoted cell functionality | 46 | |
| Coated either with a single coat of D-α-tocopheryl polyethylene glycol 1000 succinate; or a double coating of L-3,4-dihydroxyphenylalanine (L-DOPA) and human collagen type IV | Human embryonic kidney cells 293 (HEK 293) | Effective removal of uremic toxins, such as urea, creatinine, and phosphorus, to a significantly greater extent than the commercial PS membrane | 49 | |
| PS-50, was coated with L-DOPA and human collagen type IV | Conditionally immortalised proximal tubule epithelial cell (ciPTEC) line | Promoted water permeability and cell monolayer formation, as well as retained proteins such as bovine serum albumin and immunoglobulin G | 51 | |
| Blended with polyvinyl pyrrolidinone K90 (PVP) and made porous by a phase separating micro-moulding technique | ciPTEC | Different topographical arrangements of PS were found to have the ability to influence cell orientation and morphology, defined by the size and gaps of micro-features that were distributed on the membrane | 53 | |
| Poly-ε-caprolactone (PCL) | Electrospinning: random, aligned, and cryogenic and plasma treated | Human kidney primary epithelial (RC-124) cells | Growth was improved with larger diameters of the fibres, presumably it makes the scaffold to have a higher degree of porosity | 58 |
| Co-synthesised with polyethylene glycol (PEG) to produce PCL-PEG-PCL as coating on PCL | Embryonic kidney cells | Promoted three times higher cell growth than non-coated PCL, with no cytotoxicity response | 60 and 61 | |
| Polylactic acid (PLA) | Electrospinning with different fibre diameters | Rodent primary kidney cells; proximal tubular cells, collecting duct cells, podocytes, and glomerular endothelial cells | Larger diameter fibres, around 3.30 ± 0.17 μm, compared to smaller ones around 0.88 ± 0.16 μm supported cell proliferation proven by the highest DNA content after three and seven days of culture | 67 |
| Coaxial electrospinning with polyvinyl alcohol (PVA) | HEK 293 | Neat PLA still performed as the best scaffold supporting up to 75% cell viability, compared to coaxial electrospun PLA/PVA fibres supporting only 35–40% cell viability | 68 | |
| Polyglycolic acid (PGA) | Fabricated with fibrin gel | Conditionally immortalized human glomerular cells; podocytes and glomerular endothelial cells | Co-cultures exhibited an interesting self-assembly behaviour besides displaying good proliferation and cell adhesion patterns | 40 |
| Expression of collagen IV was observed, a key glomerular basement membrane (GBM) | ||||
| Poly(lactic-co-glycolic) acid (PLGA) | Porous PLGA scaffold containing magnesium hydroxide as an anti-inflammatory agent and porcine renal extracellular matrix | Nephrectomised mouse kidney | Regeneration of the glomerulus, was observed with restoration of kidney function in the mouse model | 73 |
| Silicon | Nanopore membrane with different pore sizes ranging from 10 nm to 500 nm as a haemofilter | Human cortical tubular epithelial cells (HCTC) | Allowed cell differentiation with cilia and tight junction formations | 76 |
| Natural polymers and biopolymers | ||||
| Cellulose | Cellulose membranes from regenerated cellulose sourced from cotton blended with polysulphone | — | Exhibited less biocompatibility and more immune response than polysulphone | 73 and 74 |
| The membrane replacement did not contribute to any adverse reactions for patients undergoing haemodialysis | ||||
| Fabrication of cellulose into cellulose triacetate and cellulose diacetate | — | Reduced platelet activation | 75 and 76 | |
| Nanofibrillar cellulose (NFC) hydrogel | Primary embryonic metanephric mesenchymal cells of murine source | Demonstrated a chemically induced nephrogenesis of the organoid | 77 | |
| Decellularised spinach and chive leaves coated with L-3,4-dihydroxyphenylalanine (L-DOPA) | ciPTEC | Not favourable for fostering transepithelial solute exchange due to the micro-anatomical structure of the scaffold providing a lack of permeability | 80 | |
| Produced by fermentation of Gluconacetobacter saccharivorans LMG 158, in parallel fed with D-glucose and cultured with the presence of carboxymethyl cellulose sodium salt and hydroxyapatite for in situ incorporation | HEK 293 | High cellular viability up to 97.2% | 81 | |
| Silk | Solvent-casted without any modification | Kidney organoids from induced pluripotent stem cells (iPSC) | Supported differentiation into epithelial cells from kidney progenitor cells with nephron markers | 89 |
| Showed vascularisation and induced mesenchymal cell proliferation within the scaffold under the renal capsule | ||||
| Aligned silk sponge by directional freezing; introduced in a perfusion bioreactor | Adult proximal tubule cells | Induce cell polarity | 90 | |
| Upregulation of key proximal tubule markers, especially SLC9A3, a sodium-hydrogen exchanger protein, was observed in the perfused condition | ||||
| Porous three-dimensional cylindrical silk scaffold; introduced in a perfusion bioreactor | Murine kidney epithelial and fibroblast cells in collagen-Matrigel matrix | Sustainable tissue model was generated with stability for up to six weeks for both healthy and diseased cells due to the low degradation property of silk | 91 | |
| Allowed for tissue morphogenesis that much better mimicked what is seen in vivo | ||||
| 3D printing technique generated a porous silk scaffold | Normal and polycystin-1 silenced murine inner medullary collecting duct cells encapsulated in collagen-Matrigel matrix | There are autocrine signalling loops in the silenced Pkd1 cells | 92 | |
| Unusual matrix deposition and changes in the integrin-β1 protein subunit | ||||
| Higher rate of cystogenesis was observed in the tissue | ||||
| Electrospinning; laminin-functionalised silk sheet | Podocytes derived from iPSC | Demonstrated maturation of the cells | 93 | |
| Expression of podocyte-specific markers such as podocin and nephrin | ||||
| Sustained for up to two weeks | ||||
| Bio-based hydrogel | ||||
| Extracellular matrix (ECM) | Recolonising decellularised kidneys | Primary renal cells, iPSC, embryonic stem cells, and tubular cells | Supported cell attachment and growth, as well as allowed urine production | 97–106 |
| Incorporated kidney-derived ECM within a PLGA 3D scaffold with magnesium hydroxide | Human renal cortical epithelial cells (HRCEpC) | Regeneration of renal glomerular tissue with a low inflammatory response | 73 and 112 | |
| Decellularised kidney ECM (dKECM) electrospinning with PCL | HK-2 | Promoted the formation of a tight junction, brush-border microvilli and cell polarisation | 113 and 114 | |
| Cellular metabolic activity, proliferation and protein content increased with an increase in dKECM concentrations (30, 50 and 70%) | ||||
| Expression of zona occludens-1 was revealed on the dKECM-containing membranes but not on pure PCL membranes | ||||
| ECM “tissue paper” by suspension casting from ECM ink | Human mesenchymal stem cells | Supported cell adhesion, viability, and proliferation over four weeks | 115 | |
| Matrigel | Rat mesonephric duct (Wolffian duct) cells | Supported the branching of an isolated cellular bud | 96 | |
| Photo-cross-linkable porcine kidney ECM bioink by introducing methacrylic components along the ECM fibres | Heterogeneous human primary kidney cell mixture | The cells-maintained kidney-specific phenotype and genotype, as well as forming tubular and glomerulus-like structure | 116 | |
| Formulated with gelatin, hyaluronic acid and glycerol | ||||
| ECM bioink produced with sodium alginate crosslinked by calcium chloride, and printed using by coaxial 3D cell-printing technique | Human renal proximal tubular cells (RPTEC), stem cells and human umbilical vein endothelial cells (HUVEC) | Long term graft survival was observed with expression of functional marker such as AQP1 and VE-cadherin | 117 | |
| Collagen | Collagen type I extracted from the scales of Egyption Nile Tilapia | Baby hamster kidney (BHK-21) cell line | No toxicity effects observed, even across different collagen concentrations | 119 |
| Collagen type I extracted from the swim bladder of grass carp | Scaffold introduced within nephrectomised rat kidneys | Promoted healing and kidney cell growth, led to the regeneration of damaged tubular structures and restoration of cellular metabolic function | 120 | |
| Protein functionalised with methacrylic anhydride and blended with chondroitin sulphate | ||||
| Rehydrated collagen vitrigel, a vitrificated collagen type I | Co-culture growth of glomerular epithelial cells with renal mesangial cells | Promoted the polarisation of cells observed in in vivo glomeruli | 122 | |
| Collagen-Matrigel matrix | Mixed neonatal rat renal cells | Fostered the self-assembly of cells, with tube-like structure containing CK18-positive cells and tuft-like structure Flk-1-positive cells. | 123 | |
| Collagen type I coated on a PCL cast, later the PCL was washed away with acetone to form a hollow collagen scaffold | MS-1 endothelial cells | Good cell attachment and formation of endothelium layer | 124 | |
| Gelatin | Gelatin-based hydrogel with hyaluronic acid | Human renal progenitor cell; injected under the renal cortex of mice with high serum IgA (ddY or HIGA mice) | Dcreased expression of pro-inflammatory and pro-fibrotic components was observed, increased expression of anti-inflammatory genes was observed and much reduced immunoglobulin A | 126 |
| Gelatin microcryogel | Murine pluripotent embryonic stem cells | The plasma creatinine levels decreased by 30–40% | 127 | |
| The plasma urea nitrogen decreased by 20–26% after 12 weeks | ||||
| There was a marked reduction in glomerulosclerosis and tubular injury | ||||
| Gelatin microcryogel | Mesenchymal stem cells | Decreased fibrosis, promoted antitubular inflammation and suppressing CKD progression | 128 | |
| Enzymatically crosslinked gelatin by gelatin transglutaminase to link glutamine and lysine groups | Podocytes | Expressed genes and proteins that reflect their specificity, differentiation, and functionality | 129 | |
| Microspheres crosslinked by a carbodiimide-based crosslinker | Rat kidney cells were cultured and injected into rat kidney parenchyma | Beads with a lower degree of crosslinking were more susceptible to degradation, producing good cell performance without inducing fibrosis | 35 | |
| Electrospinning formulated with a range of gelatin and acetic acid concentration ratios | HEK 293 | The electrospun fibres spun using 25% acetic acid exhibited the highest cell viability of up to 90% | 130 | |
| Gelatin-fibrinogen matrix housed in a microchannel formed by printing Pluronic F127 as fugitive ink | Renal proximal tubular cell | Maintained the culture for over two months | 131 | |
| Clear epithelial morphology and functionality comparable to those in 2D structure | ||||
| Gelatin as the main bioink component, with alginate and fibrinogen | HEK293FT | Maintained the viability of the cells by more than 90% within the hydrogel matrix | 132 | |
| Alginate | Alginate hydrogel with decellularised porcine kidney ECM crosslinked with calcium chloride | Rat renal progenitor cells | In vivo injection of the progenitor cells encapsulated within the hydrogel stimulated early-stage healing by accumulating M1 and M2 macrophages, along with hydrogel degradation over 21 days | 136 |
| Alginate microspheres coated with poly-L-lysine hydrogel | Mesenchymal stem cells injected into the damaged kidney | No inflammation and fibrosis | 137 and 138 | |
| No significant change in renal function in terms of concentration of creatinine and urea in plasma | ||||
| Thiolene functionalisation by norbornene functionalised alginate, mixed with PEG | iPSC | Suppressed the abnormal collagen type I α1 and α-smooth muscle actin (αSMA) production observed during fibrosis | 139 | |
| Oxidised alginate | iPSC | Promoting most kidney cellular segment development, tubule polarisation and cilia formation | 141 | |
| Eliminated early marker of renal fibrosis | ||||
| AG-10™ Matrix alginate and alginate from brown algae; printing of concentric tubular designs using a core–shell printhead | Primary murine tubular endothelial cells ((pmTECs), with HUVEC cells | High cell viability and metabolic activity in an appropriate arrangement led to the production of a proximal tubule wrapped by endothelial cells | 142 | |
| Synthetic hydrogels | ||||
| Polyethylene glyol | Polyethylene glycol dimethacrylate (PEGDA) and 1.5% thiol-modified carboxymethylated hyaluronic acid (CMHA-S) | Immortalized porcine LLC-PK1 renal proximal tubule epithelial cells HEK 293 and RPTEC | Stable for up to six weeks, relevant for a drug screening model focusing on nephrotoxic effects | 144 and 145 |
| Gelatin methacryloyl (GelMA) | No modification | iPSC-derived kidney organoids | Supported the development of both distal and proximal tubular structures as well as the glomerulus | 146 |
| Upregulation of nephron-related genes, including PAX8, NPHS2, NPHS1, SLC3A1 and AQP1 was observed | ||||
Conclusion and future prospective in kidney tissue engineering
Kidney tissue engineering is rapidly growing with the aim of producing the closest imitation to an organ that performs haemofiltration and complements biochemical processes to restore the innate physiological balance. Any tissue engineering attempt acknowledges the importance of creating a suitable base for regenerating functional tissue, whether it provides mechanical integrity to support appropriate cell proliferation and differentiation or creates a viable microenvironment; both are equally important.Suitability of polymers and hydrogels
Polymers and hydrogels are common materials used for kidney tissue engineering, widely tailored to optimise using the combination of multiple materials to achieve the best composition and formulation. Polymers, for instance, need to be appropriate for soft tissue engineering applications, which is important for a kidney. A material that is too stiff may affect proper cell development, as observed in the culture of podocytes.129 A bioactive substrate that supports growth and differentiation, is a major aspect of the material composition. In most cases, ECM derived from the kidney or Matrigel91,92,96 have been extensively utilised. Using other bioactive components such as hyaluronic acid116,126,144 and fibrin40,131,132 has also created a better renal cell growth environment. Hence, work carried out shows that renal tissue engineering is feasible in generating a cellularly functional kidney tissue that can perform the intended function within the human body. Silk has a special ability to support kidney cell growth without any further modification as described in this review.89–92Each material category offers specific benefits and addresses different aspects in kidney tissue engineering. Hence, to state that one type of material is superior to another would be inaccurate. They complement each other and hence, multi-material structures are a way forward to creating the best material formulation.
Challenges in developing bioartificial kidney
A step forward will be the use of these materials in a medical setting, either developing a practical and effective regenerative therapeutic approach to repair partially degenerated kidneys or a functional bioartificial kidney to replace damaged kidneys. For bioartificial kidneys, the renal cell biology needs complement the physical aspect of a natural kidney with fluid flow and dynamics, to adequately address the functionality and stability of the cells. The design needs to address the intricacy of the natural kidney architecture, to allow proper functionality. Further, the challenge continues with the need to sustain the cells by integration within the patient's physiological system This is also accompanied by immunological issues. Also, the bioartificial kidney unit should last for a significant amount of time to avoid frequent interventions which would still affect the patient's quality of life. Finally, regulatory issues for the unit's viability, reliability, and safety need to be addressed to allow patients to have a clinically approved approach. This endeavour is thus challenging but not unrealistic, given that some prototypes have already been used in animal trials,17,36,38 suggesting that they only need to be further refined for a human clinical trial.Author contributions
S. M. D. S. M.: conceptualisation, resources, visualisation, writing – original draft. G. W.: conceptualisation, writing – reviewing & editing, supervision. I. R.: conceptualisation, project administration, writing – reviewing & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. M. D. S. M. acknowledges funding by a scholarship programme, ‘Program Pelajar Cemerlang’ (Excellent Student Programme) by The Public Service Department, The Government of Malaysia. IR acknowledges funding by the EPSRC funded project (Grant no. EP/V012126/1) “SEE MORE MAKE MORE: Secondary Electron Energy Measurement Optimization for Reliable Manufacturing of Key Materials: Opportunities, Realisation & Exploitation (SM3)”. Authors also acknowledge Dr Santosh S. V. Tetali for her kind insights and inputs on kidney diseases from medical perspective for this review write-up.References
- N. K. Foundation and Global Facts About Kidney Disease, 2015. https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease, (accessed 3/9/2020, 2021).
- V. A. Luyckx, M. Tonelli and J. W. Stanifer, Bull. W. H. O., 2018, 96, 414–422D CrossRef PubMed
.
- K. Makris and L. Spanou, Clin. Biochem. Rev., 2016, 37, 85–98 Search PubMed
.
- A. L. Magno, L. Y. Herat, R. Carnagarin, M. P. Schlaich and V. B. Matthews, Biomedicines, 2019, 7, 19 CrossRef CAS PubMed
.
- S. Peerapornratana, C. L. Manrique-Caballero, H. Gómez and J. A. Kellum, Kidney Int., 2019, 96, 1083–1099 CrossRef PubMed
.
-
S. Spiliopoulos, M. Tsitskari, A. H. Holden and E. Brountzos, in Image-Guided Interventions, ed. M. A. Mauro, K. P. Murphy, K. R. Thomson, A. C. Venbrux and R. A. Morgan, Elsevier, Boston, 3rd edn, 2020, pp. 263–270. DOI:10.1016/B978-0-323-61204-3.00031-2
.
- H. K. Eltzschig and T. Eckle, Nat. Med., 2011, 17, 1391–1401 CrossRef CAS PubMed
.
-
J. Robinson, What Are Autoimmune Disorders?, 2020. https://www.webmd.com/a-to-z-guides/autoimmune-diseases, (accessed 6 Jun, 2021) Search PubMed
.
- A. S. Levey and J. Coresh, Lancet, 2012, 379, 165–180 CrossRef PubMed
.
- U. Department of Health and Human Services, High Blood Pressure & Kidney Disease, 2020, https://www.niddk.nih.gov/health-information/kidney-disease/high-blood-pressure, (accessed 6 June, 2021).
- R. Z. Alicic, M. T. Rooney and K. R. Tuttle, Clin. J. Am. Soc. Nephrol., 2017, 12, 2032 CrossRef CAS PubMed
.
- A. D. Salama and B. Caplin, J. Rheumatol., 2020, 47, 1303–1304 CrossRef PubMed
.
- C. Bergmann, L. M. Guay-Woodford, P. C. Harris, S. Horie, D. J. M. Peters and V. E. Torres, Nat. Rev. Dis. Primers, 2018, 4, 50 CrossRef PubMed
.
- S. Klahr, Intern. Med., 2000, 39, 355–361 CrossRef CAS PubMed
.
-
N. R. Aeddula and K. M. Baradhi, in StatPearls, Treasure Island (FL), 2021 Search PubMed
.
- H. D. Humes, D. Buffington, A. J. Westover, S. Roy and W. H. Fissell, Pediatr. Nephrol., 2014, 29, 343–351 CrossRef PubMed
.
- H. D. Humes, W. H. Fissell, W. F. Weitzel, D. A. Buffington, A. J. Westover, S. M. MacKay and J. M. Gutierrez, Am. J. Kidney Dis., 2002, 39, 1078–1087 CrossRef PubMed
.
- M. R. Hammerman, Kidney Int., 2003, 63, 1195–1204 CrossRef PubMed
.
- Q. Al-Awqati and J. A. Oliver, Kidney Int., 2002, 61, 387–395 CrossRef PubMed
.
-
J. F. Maher, Replacement of Renal Function by Dialysis: A Textbook of Dialysis, Springer Netherlands, 1989 Search PubMed
.
- R. Khanna, Am. J. Kidney Dis., 2017, 69, 461–472 CrossRef PubMed
.
- K. L. Johansen, G. M. Chertow, R. N. Foley, D. T. Gilbertson, C. A. Herzog, A. Ishani, A. K. Israni, E. Ku, M. Kurella Tamura, S. Li, S. Li, J. Liu, G. T. Obrador, A. M. O'Hare, Y. Peng, N. R. Powe, N. S. Roetker, W. L. St Peter, K. C. Abbott, K. E. Chan, I. H. Schulman, J. Snyder, C. Solid, E. D. Weinhandl, W. C. Winkelmayer and J. B. Wetmore, Am. J. Kidney Dis., 2021, 77, A7–a8 CrossRef PubMed
.
- A. Hayat, D. Haria and M. O. Salifu, Patient Prefer. Adherence, 2008, 2, 195–200 Search PubMed
.
- N. J. Brown and D. E. Vaughan, Circulation, 1998, 97, 1411–1420 CrossRef CAS PubMed
.
-
P.-T. T. Pham, P.-M. T. Pham, S. V. Pham, P.-A. T. Pham and P.-C. T. Pham, Diabetes, metabolic syndrome and obesity: targets and therapy, 2011, pp. 175–186 Search PubMed
.
- N. Anandakrishnan and E. U. Azeloglu, Nat. Rev. Nephrol., 2020, 16, 623–624 CrossRef PubMed
.
- B. S. Freedman and B. Ratner, ACS Cent. Sci., 2019, 5, 380–382 CrossRef CAS PubMed
.
- A. F. Tarantal and C. A. Batchelder, Woodhead Publ. Ser. Biomed., 2019, 477–492, DOI:10.1016/B978-0-08-102561-1.00020-8
.
- K. Jansen, C. C. L. Schuurmans, J. Jansen, R. Masereeuw and T. Vermonden, Curr. Pharm. Des., 2017, 23, 3845–3857 CrossRef CAS PubMed
.
- D. R. Abrahamson, P. L. St John, D. J. Pillion and D. C. Tucker, Lab. Invest., 1991, 64, 629–639 CAS
.
- R. P. Foglia, J. DiPreta, M. B. Statter and P. K. Donahoe, Ann. Surg., 1986, 204, 402–410 CrossRef CAS PubMed
.
- M. B. Statter, K. J. Fahrner, E. M. Barksdale, D. E. Parks, R. A. Flavell and P. K. Donahoe, Transplantation, 1989, 47, 651–660 CrossRef CAS PubMed
.
- B. Dekel, H. Marcus, B.-H. Herzel, W. O. Böcher, J. H. Passwell and Y. Reisner, Transplantation, 2000, 69, 1470–1478 CrossRef CAS PubMed
.
- A. S. Woolf, A. Hornbruch and L. G. Fine, Am. J. Kidney Dis., 1991, 17, 611–614 CrossRef CAS PubMed
.
- M. A. Serban, T. Knight, R. G. Payne, J. Basu, E. A. Rivera, N. Robbins, D. Mccoy, C. Halberstadt, D. Jain and T. A. Bertram, Biotechnol. Appl. Biochem., 2014, 61, 75–81 CrossRef CAS PubMed
.
- K. A. Johnston, A. J. Westover, A. Rojas-Pena, D. A. Buffington, C. J. Pino, P. L. Smith and H. D. Humes, J. Tissue Eng. Regener. Med., 2017, 11, 3048–3055 CrossRef CAS PubMed
.
- T. Souma, N. Suzuki and M. Yamamoto, Front. Physiol., 2015, 6, 167 Search PubMed
.
- W. H. Fissell, J. Kimball, S. M. MacKay, A. Funke and H. D. Humes, Ann. N. Y. Acad. Sci., 2001, 944, 284–295 CrossRef CAS PubMed
.
- M. R. Pollak, S. E. Quaggin, M. P. Hoenig and L. D. Dworkin, Clin. J. Am. Soc. Nephrol., 2014, 9, 1461–1469 CrossRef PubMed
.
- J. Tuffin, M. Burke, T. Richardson, T. Johnson, M. A. Saleem, S. Satchell, G. I. Welsh and A. Perriman, Adv. Healthc. Mater., 2019, 8, 1–11 CrossRef
.
- A. Little, A. M. Wemyss, D. M. Haddleton, B. Tan, Z. Sun, Y. Ji and C. Wan, Polymer, 2021, 13, 2458 CAS
.
- A. T. C. R. Silva, B. C. O. Cardoso, M. E. S. R. e Silva, R. F. S. Freitas and R. G. Sousa, J. Biomater. Nanobiotechnol., 2015, 6, 8 CrossRef
.
- A. Igder, S. Pye, A. H. Mohammed Al-Antaki, A. Keshavarz, C. L. Raston and A. Nosrati, RSC Adv., 2020, 10, 14761–14767 RSC
.
- Y. Koga, H. Fujieda, H. Meguro, Y. Ueno, T. Aoki, K. Miwa and M. Kainoh, Artif. Organs, 2018, 42, E246–e258 CrossRef CAS PubMed
.
- W. H. Fissell, J. Kimball, S. M. MacKay, A. Funke and H. D. Humes, Ann. N. Y. Acad. Sci., 2001, 944, 284–295 CrossRef CAS PubMed
.
- C. Shen, Q. Meng and G. Zhang, Biotechnol. Bioeng., 2013, 110, 2173–2183 CrossRef CAS PubMed
.
- S. Yamanaka and T. Yokoo, Stem Cells Int., 2015, 2015, 724047 Search PubMed
.
- I. G. Wenten, P. T. P. Aryanti, K. Khoiruddin, A. N. Hakim and N. F. Himma, J. Membr. Sci. Res., 2016, 2, 78–89 Search PubMed
.
- A. Modi, S. K. Verma and J. Bellare, Colloids Surf., B, 2018, 167, 457–467 CrossRef CAS PubMed
.
- A. Saito, Artif. Organs, 2004, 28, 58–63 CrossRef PubMed
.
- C. M. S. Schophuizen, I. E. De Napoli, J. Jansen, S. Teixeira, M. J. Wilmer, J. G. J. Hoenderop, L. P. W. Van den Heuvel, R. Masereeuw and D. Stamatialis, Acta Biomater., 2015, 14, 22–32 CrossRef CAS PubMed
.
- L. Vogelaar, J. N. Barsema, C. J. M. van Rijn, W. Nijdam and M. Wessling, Adv. Mater., 2003, 15, 1385–1389 CrossRef CAS
.
- F. Hulshof, C. Schophuizen, M. Mihajlovic, C. van Blitterswijk, R. Masereeuw, J. de Boer and D. Stamatialis, J. Tissue Eng. Regener. Med., 2018, 12, E817–E827 CAS
.
- W. Punyodom, W. Limwanich, P. Meepowpan and B. Thapsukhon, Des. Monomers Polym., 2021, 24, 89–97 CrossRef CAS PubMed
.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC
.
-
G. U. Rani and S. Sharma, in Encyclopedia of Materials: Plastics and Polymers, ed. M. S. J. Hashmi, Elsevier, Oxford, 2022, pp. 474–486, DOI:10.1016/B978-0-12-820352-1.00131-0
.
- J. Basu, C. W. Genheimer, E. A. Rivera, R. Payne, K. Mihalko, K. Guthrie, A. T. Bruce, N. Robbins, D. McCoy, N. Sangha, R. Ilagan, T. Knight, T. Spencer, B. J. Wagner, M. J. Jayo, D. Jain, J. W. Ludlow and C. Halberstadt, Cell Transplantation, 2011, 20, 1771–1790 CrossRef PubMed
.
- T. P. Burton, A. Corcoran and A. Callanan, Biomed. Mater., 2017, 13, 015006 CrossRef PubMed
.
- B. Baskapan and A. Callanan, Tissue Eng. Regener. Med., 2022, 19, 73–82 CrossRef CAS PubMed
.
- W. J. Jia, Y. C. Gu, M. L. Gou, M. Dai, X. Y. Li, B. Kan, J. L. Yang, Q. F. Song, Y. Q. Wei and Z. Y. Qian, Drug Delivery, 2008, 15, 409–416 CrossRef CAS PubMed
.
- J. Sun, X. Liu, Z. Chen, L. Jiang, M. Yuan and M. Yuan, Materials, 2022, 15, 1591 CrossRef CAS PubMed
.
- M. Gabriel, G. P. V. N. Amerongen, W. M. V. Hinsbergh and A. V. V. N. Amerongen, J. Biomater. Sci., Polym. Ed., 2006, 17, 567–577 CrossRef CAS PubMed
.
- Z. Guler, J. C. Silva and A. Sezai Sarac, Int. J. Polym. Mater. Polym. Biomater., 2017, 66, 139–148 CrossRef CAS
.
- N. Msuya, J. H. Y. Katima, E. Masanja and A. K. Temu, SciFed J. Polym. Sci., 2017, 1(1), 1–15 Search PubMed
.
- J. D. Hinchliffe, A. Parassini Madappura, S. M. D. Syed Mohamed and I. Roy, Polymer, 2021, 13, 1–47 Search PubMed
.
- J. Zan, G. Qian, F. Deng, J. Zhang, Z. Zeng, S. Peng and C. Shuai, J. Mater. Res. Technol., 2022, 17, 2369–2387 CrossRef CAS
.
- T. P. Burton and A. Callanan, Tissue Eng. Regener. Med., 2018, 15, 301–310 CrossRef CAS PubMed
.
- H. F. Alharbi, M. Luqman, K. A. Khalil, Y. A. Elnakady, O. H. Abd-Elkader, A. M. Rady, N. H. Alharthi and M. R. Karim, Eur. Polym. J., 2018, 98, 483–491 CrossRef CAS
.
- M. Ayyoob, D. H. Lee, J. H. Kim, S. W. Nam and Y. J. Kim, Fibers Polym., 2017, 18, 407–415 CrossRef CAS
.
- H. K. Makadia and S. J. Siegel, Polymer, 2011, 3, 1377–1397 CAS
.
- X. Chen, Q. Liu, J. Yang, M. Kan, R. Jin, T. Pu, Y. Yang, T. Xing, X. Meng and H. Zang, Phytother. Res., 2021, 35, 6401–6416 CrossRef CAS PubMed
.
- H. Yu, T. Lin, W. Chen, W. Cao, C. Zhang, T. Wang, M. Ding, S. Zhao, H. Wei, H. Guo and X. Zhao, Biomaterials, 2019, 219, 119368 CrossRef CAS PubMed
.
- E. Lih, W. Park, K. W. Park, S. Y. Chun, H. Kim, Y. K. Joung, T. G. Kwon, J. A. Hubbell and D. K. Han, ACS Cent. Sci., 2019, 5, 458–467 CrossRef CAS PubMed
.
- S. C. Bayliss, P. J. Harris, L. D. Buckberry and C. Rousseau, J. Mater. Sci. Lett., 1997, 16, 737–740 CrossRef CAS
.
- G. Kotzar, M. Freas, P. Abel, A. Fleischman, S. Roy, C. Zorman, J. M. Moran and J. Melzak, Biomaterials, 2002, 23, 2737–2750 CrossRef CAS PubMed
.
- W. H. Fissell, S. Manley, A. Westover, H. D. Humes, A. J. Fleischman and S. Roy, ASAIO J., 2006, 52, 221–227 CrossRef CAS PubMed
.
- B. W. Chui, N. J. Wright, J. Ly, D. A. Maginnis, T. M. Haniff, C. Blaha, W. H. Fissell and S. Roy, J. Microelectromech. Syst., 2020, 29, 762–768 CAS
.
- C. Dahmen, A. Janotta, D. Dimova-Malinovska, S. Marx, B. Jeschke, B. Nies, H. Kessler and M. Stutzmann, Thin Solid Films, 2003, 427, 201–207 CrossRef CAS
.
- T. Abitbol, A. Rivkin, Y. Cao, Y. Nevo, E. Abraham, T. Ben-Shalom, S. Lapidot and O. Shoseyov, Curr. Opin. Biotechnol, 2016, 39, 76–88 CrossRef CAS PubMed
.
- K. Jansen, M. Evangelopoulou, C. Pou Casellas, S. Abrishamcar, J. Jansen, T. Vermonden and R. Masereeuw, AAPS J., 2020, 23, 11 CrossRef PubMed
.
- C. J. Grande, F. G. Torres, C. M. Gomez and M. C. Bano, Acta Biomater., 2009, 5, 1605–1615 CrossRef CAS PubMed
.
- M. McGill, N. Raol, K. S. Gipson, S. N. Bowe, J. Fulk-Logan, A. Nourmahnad, J. Y. Chung, M. J. Whalen, D. L. Kaplan and C. J. Hartnick, Laryngoscope, 2019, 129, 2189–2194 CrossRef PubMed
.
- H. Tao, S. W. Hwang, B. Marelli, B. An, J. E. Moreau, M. Yang, M. A. Brenckle, S. Kim, D. L. Kaplan, J. A. Rogers and F. G. Omenetto, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 17385–17389 CrossRef CAS PubMed
.
- A. Bucciarelli, V. Mulloni, D. Maniglio, R. K. Pal, V. K. Yadavalli, A. Motta and A. Quaranta, Opt. Mater., 2018, 78, 407–414 CrossRef CAS
.
- S. Mohammadzadehmoghadam and Y. Dong, Front. Mater., 2019, 6, 91 CrossRef
.
- M. Farokhi, M. Aleemardani, A. Solouk, H. Mirzadeh, A. H. Teuschl and H. Redl, Biomed. Mater., 2021, 16, 022004 CrossRef CAS PubMed
.
- D. A. Gregory, M. A. Tomeh and X. Zhao, Int. J. Mol. Sci., 2021, 22(3), 1499 CrossRef PubMed
.
- J.-L. Duval, T. Dinis, G. Vidal, P. Vigneron, D. L. Kaplan and C. Egles, J. Tissue Eng. Regener. Med., 2017, 11, 354–361 CrossRef CAS PubMed
.
- A. K. Gupta, J. M. Coburn, J. Davis-Knowlton, E. Kimmerling, D. L. Kaplan and L. Oxburgh, J. Tissue Eng. Regener. Med., 2019, 13, 812–822 CrossRef CAS PubMed
.
- S. Szymkowiak, N. Sandler and D. L. Kaplan, ACS Appl. Mater. Interfaces, 2021, 13, 10768–10777 CrossRef CAS PubMed
.
- B. Subramanian, D. Rudym, C. Cannizzaro, R. Perrone, J. Zhou and D. L. Kaplan, Tissue Eng., Part A, 2010, 16, 2821–2831 CrossRef CAS PubMed
.
- B. Subramanian, W.-C. Ko, V. Yadav, T. M. DesRochers, R. D. Perrone, J. Zhou and D. L. Kaplan, Biomaterials, 2012, 33, 8383–8394 CrossRef CAS PubMed
.
- X. Mou, J. Shah, R. Bhattacharya, T. D. Kalejaiye, B. Sun, P.-C. Hsu and S. Musah, Bioengineering, 2022, 9, 188 CrossRef CAS PubMed
.
- R. Sobreiro-Almeida, R. Quinteira and N. M. Neves, Adv. Healthc. Mater., 2021, 10, 2100160 CrossRef CAS PubMed
.
-
D. L. Paz, Y. Le Meur and Y. Renaudineau, in Autoantibodies, ed. Y. Shoenfeld, P. L. Meroni and M. E. Gershwin, Elsevier, San Diego, 3rd edn, 2014, pp. 553–560, DOI:10.1016/B978-0-444-56378-1.00065-4
.
- E. Rosines, R. V. Sampogna, K. Johkura, D. A. Vaughn, Y. Choi, H. Sakurai, M. M. Shah and S. K. Nigam, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20938–20943 CrossRef CAS PubMed
.
- S. H. Choi, S. Y. Chun, S. Y. Chae, J. R. Kim, S. H. Oh, S. K. Chung, J. H. Lee, P. H. Song, G.-S. Choi, T.-H. Kim and T. G. Kwon, J. Biomed. Mater. Res., Part A, 2015, 103, 1391–1403 CrossRef PubMed
.
- M. Abolbashari, S. M. Agcaoili, M.-K. Lee, I. K. Ko, T. Aboushwareb, J. D. Jackson, J. J. Yoo and A. Atala, Acta Biomater., 2016, 29, 52–61 CrossRef CAS PubMed
.
- O. Ciampi, B. Bonandrini, M. Derosas, S. Conti, P. Rizzo, V. Benedetti, M. Figliuzzi, A. Remuzzi, A. Benigni, G. Remuzzi and S. Tomasoni, Sci. Rep., 2019, 9, 8001 CrossRef PubMed
.
- I. Ullah, J. F. Busch, A. Rabien, B. Ergün, C. Stamm, C. Knosalla, S. Hippenstiel, P. Reinke and A. Kurtz, Adv. Sci., 2020, 7, 1901198 CrossRef CAS PubMed
.
- C. Du, K. Narayanan, M. F. Leong, M. S. Ibrahim, Y. P. Chua, V. M. H. Khoo and A. C. A. Wan, Adv. Healthc. Mater., 2016, 5, 2080–2091 CrossRef CAS PubMed
.
- D. G. Leuning, F. M. R. Witjas, M. Maanaoui, A. M. A. de Graaf, E. Lievers, T. Geuens, C. M. Avramut, L. E. Wiersma, C. W. van den Berg, W. M. P. J. Sol, H. de Boer, G. Wang, V. L. S. LaPointe, J. van der Vlag, C. van Kooten, B. M. van den Berg, M. H. Little, M. A. Engelse and T. J. Rabelink, Am. J. Transplant., 2019, 19, 1328–1343 CrossRef CAS PubMed
.
- Z. Tan, A. Rak-Raszewska, I. Skovorodkin and S. J. Vainio, Cells, 2020, 9(2), 329 CrossRef CAS PubMed
.
- A. Remuzzi, M. Figliuzzi, B. Bonandrini, S. Silvani, N. Azzollini, R. Nossa, A. Benigni and G. Remuzzi, Sci. Rep., 2017, 7, 43502 CrossRef PubMed
.
- Y. Guan, S. Liu, C. Sun, G. Cheng, F. Kong, Y. Luan, X. Xie, S. Zhao, D. Zhang, J. Wang, K. Li and Y. Liu, Oncotarget, 2015, 6(34), 36126–36138 CrossRef PubMed
.
- M. Caralt, J. S. Uzarski, S. Iacob, K. P. Obergfell, N. Berg, B. M. Bijonowski, K. M. Kiefer, H. H. Ward, A. Wandinger-Ness, W. M. Miller, Z. J. Zhang, M. M. Abecassis and J. A. Wertheim, Am. J. Transplant., 2015, 15, 64–75 CrossRef CAS PubMed
.
- R. J. Nagao, J. Xu, P. Luo, J. Xue, Y. Wang, S. Kotha, W. Zeng, X. Fu, J. Himmelfarb and Y. Zheng, Tissue Eng., Part A, 2016, 22, 1140–1150 CrossRef CAS PubMed
.
- V. Magno, J. Friedrichs, H. M. Weber, M. C. Prewitz, M. V. Tsurkan and C. Werner, Acta Biomater., 2017, 55, 109–119 CrossRef CAS PubMed
.
- J. Su, S. C. Satchell, R. N. Shah and J. A. Wertheim, J. Biomed. Mater. Res., Part A, 2018, 106, 2448–2462 CrossRef CAS PubMed
.
- C. Zhou, L. Zhou, J. Liu, L. Xu, Z. Xu, Z. Chen, Y. Ge, F. Zhao, R. Wu, X. Wang, N. Jiang, L. Mao and R. Jia, Acta Biomater., 2020, 115, 250–263 CrossRef CAS PubMed
.
- J. D. O'Neill, D. O. Freytes, A. J. Anandappa, J. A. Oliver and G. V. Vunjak-Novakovic, Biomaterials, 2013, 34, 9830–9841 CrossRef PubMed
.
- E. Lih, K. W. Park, S. Y. Chun, H. Kim, T. G. Kwon, Y. K. Joung and D. K. Han, ACS Appl. Mater. Interfaces, 2016, 8, 21145–21154 CrossRef CAS PubMed
.
- R. Sobreiro-Almeida, D. R. Fonseca and N. M. Neves, Mater. Sci. Eng., C, 2019, 103, 109866 CrossRef CAS PubMed
.
- R. Sobreiro-Almeida, M. E. Melica, L. Lasagni, P. Romagnani and N. M. Neves, Acta Physiol., 2020, 230, e13491 CAS
.
- A. E. Jakus, M. M. Laronda, A. S. Rashedi, C. M. Robinson, C. Lee, S. W. Jordan, K. E. Orwig, T. K. Woodruff and R. N. Shah, Adv. Funct. Mater., 2017, 27, 1700992 CrossRef PubMed
.
- M. Ali, A. K. Pr, J. J. Yoo, F. Zahran, A. Atala and S. J. Lee, Adv. Healthc. Mater., 2019, 8, 1800992 CrossRef PubMed
.
- N. K. Singh, W. Han, S. A. Nam, J. W. Kim, J. Y. Kim, Y. K. Kim and D.-W. Cho, Biomaterials, 2020, 232, 119734 CrossRef CAS PubMed
.
- S. J. Lee, H.-J. Wang, T.-H. Kim, J. S. Choi, G. Kulkarni, J. D. Jackson, A. Atala and J. J. Yoo, Stem Cells Transl. Med., 2018, 7, 241–250 CrossRef CAS PubMed
.
- A. A. El-Rashidy, A. Gad, A. E.-H. G. Abu-Hussein, S. I. Habib, N. A. Badr and A. A. Hashem, Int. J. Biol. Macromol., 2015, 79, 618–626 CrossRef CAS PubMed
.
- H. Wu, R. Zhang, B. Hu, Y. He, Y. Zhang, L. Cai, L. Wang, G. Wang, H. Hou and X. Qiu, Chin. Chem. Lett., 2021, 32, 3940–3947 CrossRef CAS
.
- T. E. Kruger, A. H. Miller and J. Wang, Sci. World J., 2013, 2013, 812718 Search PubMed
.
- P. C. Wang and T. Takezawa, J. Biosci. Bioeng., 2005, 99, 529–540 CrossRef CAS PubMed
.
- S. H. Lu, Q. X. Lin, Y. N. Liu, Q. Gao, T. Hao, Y. Wang, J. Zhou, H. B. Wang, Z. Y. Du, J. Wu and C. Y. Wang, J. Tissue Eng. Regener. Med., 2012, 6, 786–792 CrossRef PubMed
.
- J. Huling, I. K. Ko, A. Atala and J. J. Yoo, Acta Biomater., 2016, 32, 190–197 CrossRef CAS PubMed
.
- J. M. Koli, S. Basu, B. B. Nayak, N. Kannuchamy and V. Gudipati, J. Food Sci., 2011, 76, E503–E509 CrossRef CAS PubMed
.
- S. Khunmanee, S. Y. Chun, Y.-S. Ha, J. N. Lee, B. S. Kim, W.-W. Gao, I. Y. Kim, D. K. Han, S. You, T. G. Kwon and H. Park, Tissue Eng. Regener. Med., 2022, 19, 643–658 CrossRef CAS PubMed
.
- X. D. Geng, W. Zheng, C. M. Wu, S. Q. Wang, Q. Hong, G. Y. Cai, X. M. Chen and D. Wu, China Med. J., 2016, 129, 392–398 CrossRef CAS PubMed
.
- X. Geng, Q. Hong, K. Chi, S. Wang, G. Cai and D. Wu, BioMed Res. Int., 2019, 2019, 6749326 Search PubMed
.
- M. Hu, E. U. Azeloglu, A. Ron, K.-H. Tran-Ba, R. C. Calizo, I. Tavassoly, S. Bhattacharya, G. Jayaraman, Y. Chen, V. Rabinovich, R. Iyengar, J. C. Hone, J. C. He and L. J. Kaufman, Sci. Rep., 2017, 7, 43934 CrossRef PubMed
.
- M. Erencia, F. Cano, J. A. Tornero, M. M. Fernandes, T. Tzanov, J. Macanás and F. Carrillo, J. Appl. Polym. Sci., 2015, 132(25), 1–11 CrossRef
.
- K. A. Homan, D. B. Kolesky, M. A. Skylar-Scott, J. Herrmann, H. Obuobi, A. Moisan and J. A. Lewis, Sci. Rep., 2016, 6, 34845 CrossRef CAS PubMed
.
- L. Ouyang, R. Yao, X. Chen, J. Na and W. Sun, Biofabrication, 2015, 7, 015010 CrossRef PubMed
.
- I. D. Hay, Z. Ur Rehman, M. F. Moradali, Y. Wang and B. H. Rehm, Microb. Biotechnol., 2013, 6, 637–650 CrossRef CAS PubMed
.
- A. D. Augst, H. J. Kong and D. J. Mooney, Macromol. Biosci., 2006, 6, 623–633 CrossRef CAS PubMed
.
- M. Szekalska, A. Puciłowska, E. Szymańska, P. Ciosek and K. Winnicka, Int. J. Polym. Sci., 2016, 2016, 7697031 Search PubMed
.
- T. L. Chu, G. Tripathi, M. Park, S.-H. Bae and B.-T. Lee, Int. J. Biol. Macromol., 2022, 211, 616–625 CrossRef CAS PubMed
.
- M. F. A. Goosen, G. M. O'Shea, H. M. Gharapetian, S. Chou and A. M. Sun, Biotechnol. Bioeng., 1985, 27, 146–150 CrossRef CAS PubMed
.
- E. Trouche, S. G. Fullana, C. Mias, C. Ceccaldi, F. Tortosa, M. H. Seguelas, D. Calise, A. Parini, D. Cussac and B. Sallerin, Cell Transplantation, 2010, 19, 1623–1633 CrossRef CAS PubMed
.
- T. Geuens, F. A. A. Ruiter, A. Schumacher, F. L. C. Morgan, T. Rademakers, L. E. Wiersma, C. W. van den Berg, T. J. Rabelink, M. B. Baker and V. L. S. LaPointe, Biomaterials, 2021, 275, 120976 CrossRef CAS PubMed
.
- S. Reakasame and A. R. Boccaccini, Biomacromolecules, 2018, 19, 3–21 CrossRef CAS PubMed
.
- F. A. A. Ruiter, F. L. C. Morgan, N. Roumans, A. Schumacher, G. G. Slaats, L. Moroni, V. L. S. LaPointe and M. B. Baker, Adv. Sci., 2022, 9, 2200543 CrossRef CAS PubMed
.
- G. Addario, S. Djudjaj, S. Farè, P. Boor, L. Moroni and C. Mota, Bioprinting, 2020, 20, e00108 CrossRef
.
- A. Z. Unal and J. L. West, Bioconjugate Chem., 2020, 31, 2253–2271 CrossRef CAS PubMed
.
- A. I. Astashkina, B. K. Mann, G. D. Prestwich and D. W. Grainger, Biomaterials, 2012, 33, 4700–4711 CrossRef CAS PubMed
.
- A. I. Astashkina, B. K. Mann, G. D. Prestwich and D. W. Grainger, Biomaterials, 2012, 33, 4712–4721 CrossRef CAS PubMed
.
- S. Clerkin, J. K. Wychowaniec, K. Singh, J. L. Davis, N. J. Treacy, I. Krupa, D. F. Brougham and J. Crean, FASEB J., 2022, 36, R3064 Search PubMed
.
- S. C. Satchell, C. H. Tasman, A. Singh, L. Ni, J. Geelen, C. J. von Ruhland, M. J. O'Hare, M. A. Saleem, L. P. van den Heuvel and P. W. Mathieson, Kidney Int., 2006, 69, 1633–1640 CrossRef CAS PubMed
.
- V. Volkov, A. V. Ferreira and A. Cavaco-Paulo, Macromol. Mater. Eng., 2015, 300(12), 1199–1216 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |