Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of a glassy carbon electrode modified with saffron conjugated silver nanoparticles for the sensitive and selective electroanalytical determination of amoxicillin in urine samples
Christina
Sarakatsanou
 ,
Sophia
Karastogianni
* and
Stella
Girousi
,
Sophia
Karastogianni
* and
Stella
Girousi
 *
*
Analytical Chemistry Laboratory, School of Chemistry, Faculty of Sciences, 54124 Thessaloniki, Greece. E-mail: christies@chem.auth.gr; karastos@thea.auth.gr; girousi@chem.auth.gr; Fax: +3023-1099-7719; Tel: +3023-1099-7722
First published on 23rd August 2023
Abstract
Determination of antibiotics is crucial in order to assess their potential impacts on human health and the environment. This study aimed to develop a modified glassy carbon electrode with saffron conjugated silver nanoparticles for the determination of amoxicillin antibiotic in urine samples. The modified electrode was prepared by electrodeposition of silver nanoparticles on the electrode surface, followed by deposition of amoxicillin on the surface. The electrochemical behavior of the modified electrode was studied by cyclic voltammetry and square wave voltammetry. The results showed that the modified electrode exhibited enhanced electrocatalytic activity toward the oxidation of amoxicillin. The calibration curve was linear in the concentration range from 1.273 × 10−4 g L−1 to 2.217 × 10−3 g L−1, with a high linear correlation coefficient of 0.9998. The detection limit was determined to be 4.199 × 10−5 g L−1. The precision of the sensor was adequate, with relative standard deviations of 4.3% and 4.0% for AMX concentrations of 9.199 × 10−5 g L−1 and 1.194 × 10−4 g L−1, respectively. The modified electrode was then applied to the determination of amoxicillin in urine samples. The method showed linearity over the amoxicillin concentration range from 0.00 to 2.00 × 10−4 g L−1, with a detection limit of 9.739 × 10−6 g L−1, indicating the potential of the modified electrode for the determination of amoxicillin in biological samples. Overall, the modified glassy carbon electrode with silver nanoparticles showed very promising results for the sensitive and selective determination of amoxicillin in urine samples.
1. Introduction
Medications like antibiotics are necessary to cure many illnesses brought about by harmful microorganisms. However, there's worry over bacterial resistance and the environmental impact caused by these drugs since they're often used in both healthcare and food production. They are considered “pseudopersistent” contaminants because they are frequently entering the ecosystem.1Antibiotics are a highly successful form of treatment that has saved countless lives and helped to control many infectious diseases that once plagued humans for centuries. When antibiotics were first introduced in the 1940s, they were incredibly effective at eliminating harmful bacteria, leading many to believe that infectious diseases would eventually be eradicated. However, the emergence of antibiotic-resistant pathogens, particularly multi-drug-resistant bacteria, in recent decades has highlighted our limited understanding of the evolutionary and ecological processes taking place in microbial ecosystems. Microbial populations have a vast range of metabolic diversity, allowing them to develop protective mechanisms against selective pressures from their environment, including antibiotics.2
Moreover, the use of antibiotics in animal agriculture has been linked to the emergence of antibiotic-resistant strains of pathogenic bacteria that can affect humans.3 In addition, bacteria can develop resistance to both veterinary and human antibiotics that have similar structures. Today we have a better understanding of antibiotics and antibiotic resistance, and it has become clear that monitoring the levels of antibiotics is of great importance. Monitoring the levels of antibiotics in the environment is crucial to assess their potential impacts on human health and the environment.2 Therefore, there is a need for sensitive and selective methods for the determination of antibiotic residues in various samples. Analyzing antibiotics can be challenging due to the complexity of the samples being analyzed and the typically low concentrations at which these substances are found in these kinds of samples. This requires the use of highly sensitive analytical methods for monitoring these compounds at low concentration levels.
Several separation methods have been reported for the sensitive determination of antibiotics, such as high performance liquid chromatography (HPLC),4 capillary electrophoresis (CE),5 and thin layer chromatography (TLC),6 which are often used in conjunction with diode array detectors (DAD)7 and mass spectrometry (MS) detectors8 to accurately determine antibiotics.
Moreover, the separation methods mentioned above are usually combined with preconcentration techniques, such as solid phase extraction,4,8 and liquid–liquid extraction (LLE),9 which are often used before detection with liquid chromatography. However, the direct injection of samples into high-performance liquid chromatography systems has become a widely utilized technique.
While chromatography is a valuable tool for identifying antibiotics, it has its limitations, including the expenses associated with equipment and time. Similarly, other highly precise methods for detecting antibiotics, such as direct assay, fluorescence immunoassay, enzyme-linked immunosorbent assay, and capillary electrophoresis, require sophisticated sample preparation and skilled professionals, which restricts their widespread use.10
So, there's an urgent need for more precise, straightforward, and cost-effective methods to detect antibiotics. Electrochemical sensors have the potential to be used as a screening tool to quickly estimate antibiotic contamination in different samples. Electroanalytical chemistry is a reliable technique that provides high sensitivity and selectivity for determining antibiotics in complex matrices. To create effective electrochemical sensors for therapeutic drug monitoring (TDM) that are also affordable, it's crucial to select appropriate electrode and coating materials for functionalization purposes.11
Modified electrodes have demonstrated successful application in various fields such as energy production and analytical chemistry. There are various types of modified electrodes, such as carbon paste electrodes, chemically modified electrodes, enzyme modified electrodes, microelectrodes and nanoparticle-modified electrodes, that have been particularly effective in detecting low levels of amino acids, peptides, proteins, alcohols, and sugars in electrochemical experiments, as well as in studying inorganic ions. However, the long-term stability and ability to restore activity after surface contamination can be limiting factors.12
Over the last few years, modified electrodes have been shown to be effective in a range of electroanalytical techniques, including amperometry and voltammetry, and can detect a diverse selection of antibiotics. Nanoparticle-mediated modification of electrodes has yielded promising results in the electroanalytical determination of various antibiotics, such as penicillins, cephalosporins, tetracyclines, and quinolones. Recent studies have demonstrated the use of nanomaterials, including carbon nanotubes13 and graphene oxide,14 as electrode modifiers, which have resulted in improved electrocatalytic properties, as well as increased sensitivity and selectivity in electrochemical sensors. For instance, Feizollahi et al.14 developed a new approach for detecting sulfamethazine (SMZ) in cow milk, by using a glassy carbon electrode that was modified with graphene oxide and decorated with Cu–Ag core–shell nanoparticles. Nanoparticles can create additional active sites for the adsorption of antibiotics, increase the electrochemical surface area, and enhance electron transfer between the analyte and the electrode, resulting in superior sensitivity, selectivity, and stability, compared to unmodified electrodes.
These modified electrodes have been applied successfully to the determination of a broad range of antibiotics, including chloramphenicol,15 doxorubicin16 pyrazinamide,17 streptomycin18 and amoxicillin.19 Some examples of electrode surfaces modified with nanoparticles that have been used in the electroanalytical determination of antibiotics are shown on Table 1.
These are just a few examples of nanoparticle-modified electrode surfaces that have been used for the electroanalytical determination of antibiotics. There are more examples of electrode surfaces modified with nanoparticles for the sensitive and selective electroanalytical determination of antibiotics. For instance, Shun Liu et al.26 developed a sensor using a nanocomposite of reduced graphene oxide and silver nanoparticles to detect the antibiotic chloramphenicol. This sensor was proved to be reproducible, stable, and selective over similar interfering substances in order to accurately detect chloramphenicol in milk samples. Cesarino et al.13 also reported the preparation of a paraffin composite electrode based on multi-walled carbon nanotubes (MWCNT) modified with antimony nanoparticles (SbNPs). The sensor was used to detect two antibiotics, sulfamethoxazole and trimethoprim, using differential pulse voltammetry in natural water samples. The sensor's structure and electrochemical properties were studied using field emission gun scanning electron microscopy and cyclic voltammetry, respectively.
These examples demonstrate the potential of using nanoparticles to modify electrodes surfaces for the sensitive and selective electroanalytical determination of antibiotics, but there are many more. In one study, the development of an electrochemical sensor using platinum nanoparticles on carbon (PtNPs/C) for the detection of tetracycline, is described. The sensor was first synthesized and characterized using X-ray diffraction and transmission electron microscopy. The researchers then studied how different experimental conditions, such as the amount of platinum nanoparticles and the pH of the solution, affected the sensor's behavior. They used cyclic voltammetry and differential pulse voltammetry to investigate how tetracycline is electrooxidized on the sensor and determined that the sensor was able to accurately detect tetracycline over a wide range of concentrations in urine samples. This suggests that the sensor may have potential for use in clinical analysis and quality control.28
In another study, Zhu et al. developed a copper nanoparticle-based film incorporating cationic surfactant and graphene to determine the presence of gatifloxacin and pefloxacin with differential pulse stripping voltammetry. The film surface was analyzed using SEM and EDS and found to have improved electrocatalytic properties due to the combination of copper nanoparticles, graphene and CTAB surfactants. The modified electrode showed good performance in drug detection, with linear responses at concentrations between 0.02–40 μM and 0.04–20 μM and detection limits of 0.0021 μM and 0.0025 μM, respectively. The sensors were also used to detect these drugs in shrimp and animal serum with successful results.37
Over the past few decades, various studies have reported on the use of electrochemical methods for accurately measuring the presence of antibiotics. These methods have proven to be effective in detecting these substances in a quantitative manner. Primarily, according to Dai et al.,38 gold nanoparticles (∼30–60 nm in diameter) were deposited onto the surface of glassy carbon microspheres. Later, Wang et al.39 reported the use of a tetracycline sensor, using molecularly imprinted polymer modified carbon nanotube-gold nanoparticles electrode.
In addition to their electrocatalytic properties, these nanoparticle-modified electrodes have the advantages of low cost, high sensitivity, and convenient operability, making them very promising for practical applications in the analysis of antibiotic residues. Although there are many advantages of nanoparticle-modified electrodes, there are also some challenges and limitations to their use.30 For instance, the synthesis and functionalization of nanoparticles can be difficult and time-consuming, and the stability of these materials can vary depending on the conditions of the electroanalytical measurement. Additionally, the electrochemical signal can interference from other compounds with similar chemical structures and limit the selectivity of these sensors.31,32 Thus, further research is needed to overcome potential interferences from analogues with similar chemical structures and to improve the stability and selectivity of these sensors.
The purpose of this paper is to present a novel method for the preparation of a glassy carbon electrode modified with saffron conjugated silver nanoparticles that can be used for the sensitive and selective electroanalytical determination of amoxicillin in urine samples using cyclic voltammetry and square wave voltammetry. AgNPs@Sa were used as modifiers for the glassy carbon electrode (GCE), creating the AgNPs@Sa-GCE modified electrode for amoxicillin determination. The study investigated various parameters, including medium change, amoxicillin interaction potential, interaction time, and pH. Overall, this research improved the GCE characteristics and showcased the effective use of electrochemical techniques for routine analysis. The study aims to explore the potential of this electrode in detecting amoxicillin at low concentrations in urine samples with high precision and accuracy. This research has the potential to contribute to the development of effective and affordable electroanalytical tools for therapeutic drug monitoring, particularly in cases where precise amoxicillin quantification is required for effective patient care.
2. Materials and methods
For the voltammetric experiments, a Palm Sens Model 1 potentiostat/galvanostat from Echo Chemie in the Netherlands was used, along with an ultrasonic bath (TRANSONIC 460/H) to dissolve nanoparticles and buffer solutions. The electrochemical cell used in this experiment included a platinum wire counter electrode (Metrohm, Switzerland), an Ag/AgCl reference electrode saturated with 3 mol L−1 KCl, and a glassy carbon working electrode (GCE) with an inner diameter of 3 mm and an outer diameter of 9 mm. The electrodes were used for the electropolymerization of silver nanoparticles and the deposition of amoxicillin. Weighings during the experiment were done using Sartorius type scales, Kernew 220-30014 and Denver Instrument XE-310. All procedures were carried out at ambient temperature, and the pH of all solutions was measured using a Consort C830 pH meter. Prior to each experiment, ultrapure nitrogen was used to degas the liquids by removing dissolved oxygen for 15 min. The electrochemical cells were washed and rinsed with deionized water and cleaned with dilute nitric acid.All reagents used in the experiment were of analytical grade and were used exactly as received. Tris-hydroxymethyl-aminomethane (Tris 99.8, ACS), ethyl-diamine-tetraacetic acid (EDTA, ACS reagent, 99.4–100.06), potassium dihydrogen phosphate and dipotassium hydrogen phosphate (Darmstadt, Germany) were provided by Merck. Sodium hydroxide, hydrochloric acid, and nitric acid were provided by Sigma-Aldrich (Saint Louis, MO, USA), while saffron was sourced from the local market. Amoxicillin from the Roth company, with a purity of >99.5, was used in this experiment. All aqueous solutions were prepared using bis-deionized water. The experiment aimed to synthesize silver nanoparticles and utilize them to polymerize the GCE electrode, enabling accurate determination of amoxicillin, a penicillin antibiotic used to treat bacterial infections. The initial amoxicillin solution was prepared by weighing a certain amount of amoxicillin and diluting it in deionized water to a concentration of 1 g L−1.
2.1 Synthesis of saffron-conjugated silver nanoparticles
The silver nanoparticles were synthesized using an eco-friendly approach that involved saffron as a stabilizer and reducing agent.33,34 In a 25 ml volumetric tube, saffron and silver nitrate (AgNO3) were mixed in different ratios, from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and from 1
9 and from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 9
1 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). A certain volume of caustic sodium (NaOH) was added to every mixture to adjust the pH to 10, followed by the addition of deionized water to reach the mark, and stirring for 5 minutes using a Vortex. The resulting solutions were allowed to settle for approximately 15 minutes, resulting in a color change from red-orange to brownish-yellow. The solutions were then filtered and the precipitate was washed with bis-deionized water and ethanol and left to dry at room temperature for 24 hours. In order to assess the optimal ratios and the presence of silver within the nanoparticles, the AgNPs were characterized using scanning electron microscopy (SEM) and energy-dispersive X-ray analysis (EDAX). Then, the saffron-conjugated silver nanoparticles, each from a different ratio, were mixed together.
1 (w/w). A certain volume of caustic sodium (NaOH) was added to every mixture to adjust the pH to 10, followed by the addition of deionized water to reach the mark, and stirring for 5 minutes using a Vortex. The resulting solutions were allowed to settle for approximately 15 minutes, resulting in a color change from red-orange to brownish-yellow. The solutions were then filtered and the precipitate was washed with bis-deionized water and ethanol and left to dry at room temperature for 24 hours. In order to assess the optimal ratios and the presence of silver within the nanoparticles, the AgNPs were characterized using scanning electron microscopy (SEM) and energy-dispersive X-ray analysis (EDAX). Then, the saffron-conjugated silver nanoparticles, each from a different ratio, were mixed together.
After obtaining the saffron-conjugated silver nanoparticles through the drying process, a specific amount of the solid nanoparticles was weighed (0.015 g for a concentration of 3 g L−1), added to a 5 ml volumetric flask with bi-deionized water to the mark, and subjected to ultrasonic treatment for 15 minutes to prepare it for use.
2.2 Preparation of working electrodes
In this study, a glassy carbon electrode (GCE) was utilized as the working electrode, modified with silver nanoparticles conjugated with saffron. The GCE did not require pretreatment but was polished with alumina after each modification with nanoparticles. All experiments were conducted at room temperature and prior to the voltammetric measurements, the solutions within the three-electrode cell were purged with high-purity nitrogen gas for 15 minutes to remove dissolved oxygen. The formation of the GCE involved the electropolymerization of saffron-conjugated silver nanoparticles (Sa@AgNPs) using cyclic voltammetry, which were then deposited on the surface of the GCE to create a poly-Sa@AgNPs-GCE. Cyclic voltammetry was used to polymerize the solution of AgNPs@Sa (0.1 mol L−1 acetate buffer with pH 5.6 containing 12 × 10−4 g L−1 Sa@AgNPs), sweeping the potential from −0.100 to +1.300 V for one scan cycle, with a potential step equal to 0.009 V and a potential scan rate equal to 0.035 V s−1 and the resulting poly-AgNPs@Sa were electrodeposited onto the GCE surface. The resulting GCE was utilized to detect amoxicillin (AMX) in standard solutions of known concentrations as well as in a real urine sample.2.3 Experimental procedure
The buffers which were used with the voltammetric techniques were buffers of 0.1 mol L−1 acetate (CH3COOH/CH3COONa), 0.1 mol L−1 phosphate (NaH2PO4/Na2HPO4) and 0.1 mol L−1 Tris–HCl. Determination of amoxicillin (AMX) was done using standard solutions of known AMX mass concentration, using the technique of square wave voltammetry. First, the polymeric film of silver nanoparticles with saffron (Sa@AgNPs) was immobilized on the GCE surface in a pH 5.6 acetate buffer containing 12 × 10−4 g L−1 Sa@AgNPs. The polymer film was deposited on the surface of the GCE potentiodynamically using cyclic voltammetry, scanning the potential of the working electrode between values of −0.100 and +1.300 V. The potential was scanned sequentially for one scan cycle and with a scan rate of 35 mV s−1 and potential step equal to 9 mV. Then, the shaped GCE with the polymer film of Sa@AgNPs (poly Sa@AgNPs-CPE) was rinsed with bis deionized water. The interaction of AMX with poly Sa@AgNPs-CPE was performed by immersing the shaped poly Sa@AgNPs-CPE in the AMX solubilization solution containing different mass concentrations of AMX (0.1 mol NaH2PO4/Na2HPO4 pH = 7.0) and KBr (0.05 mol L−1). Immobilization of AMX was done without potential application by stirring for a time equal to 180 s. Then, the patterned electrode was rinsed with deionized bis water and transferred to 0.1 mol L−1 acetate buffer pH 4.0 with 0.01 mol L−1 NaNO3, where the signal conversion was performed by square wave voltammetry (SWV). The potential was swept up between values of −0.300 and +1.300 V, while the potential step was equal to 8 mV, the pulse potential was equal to 30 mV, and the frequency was equal to 30 Hz.For the analysis of the urine sample, a known quantity of a standard solution was added following a protocol that involved filtering the urine through a 45 mm Millipore membrane, diluting it 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 with bis deionized water, sonication for 10 min, and refiltering through a 45 mm Millipore membrane. A 1
100 with bis deionized water, sonication for 10 min, and refiltering through a 45 mm Millipore membrane. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 dilution was then performed, followed by a 10 minute sonication. To reduce the background concentration in the blank matrices, the urine samples were diluted before adding the standard solutions. A known volume of amoxicillin standard solution was added to each of the seven volumetric flasks containing the diluted urine samples. The procedure for determining AMX in standard solutions was then followed, and a curve was constructed by adding known amounts of the standard solution, using the oxidation signal at +0.150 V of the solutions. Square voltammograms obtained were processed with the Palm Sens software filter, and baseline noise was subtracted by the program with an amplitude of 0.03. The measurements were conducted using the GCE electrode whose surface was polished before each measurement with alumina. One of our laboratory team members provided the urine samples.
25 dilution was then performed, followed by a 10 minute sonication. To reduce the background concentration in the blank matrices, the urine samples were diluted before adding the standard solutions. A known volume of amoxicillin standard solution was added to each of the seven volumetric flasks containing the diluted urine samples. The procedure for determining AMX in standard solutions was then followed, and a curve was constructed by adding known amounts of the standard solution, using the oxidation signal at +0.150 V of the solutions. Square voltammograms obtained were processed with the Palm Sens software filter, and baseline noise was subtracted by the program with an amplitude of 0.03. The measurements were conducted using the GCE electrode whose surface was polished before each measurement with alumina. One of our laboratory team members provided the urine samples.
3. Results and discussion
3.1 Study of the morphology of saffron-conjugated silver nanoparticle glassy carbon electrode
The present study employed scanning electron microscopy (SEM) to investigate the morphological characteristics of the AgNPs@Sa-GCE (Fig. 1a and b) and energy dispersive X-ray spectroscopy (EDAX) to demonstrate the presence of silver in the nanoparticles (Fig. 1c). The SEM images show that spherical-shaped Ag nanoparticles were synthesized, and Fig. 1 depicts the particles in the form of organized aggregates. Morphological differences between GCE and poly-AgNPs@Sa-GCE were observed in Fig. 1a–c, confirming the deposition of AgNPs@Sa on the surface of GCE. Particle size varied, although their precise dimensions could not be determined. Furthermore in Fig. 1c we observe a high intensity silver peak, which proves the existence of silver in the nanoparticles.3.2 Polymerization of silver nanoparticles on GCE
Polymeric film of silver nanoparticles with saffron (Sa@AgNPs) was immobilized on the GCE surface in a pH 5.6 acetate buffer containing 12 × 10−4 g L−1 Sa@AgNPs. The polymer film was deposited on the surface of the GCE potentiodynamically using cyclic voltammetry, scanning the potential of the working electrode between values of −0.100 and +1.300 V vs. Ag/AgCl. The potential was scanned sequentially for one scan cycle and with a scan rate of 35 mV s−1 and potential step equal to 9 mV. A pretreatment step was not used on GCE, which demonstrates a progressive increase in the peak current over the polymerization process. As can be seen in Fig. 2, the peak current responses of the one irreversible oxidation peak at about +0.385 V increase progressively up to the fifteenth scan cycle. Furthermore, above the fifth scan cycle, the current reduction peak disappears. This oxidation peak could be attributed to the oxidation of crocin.33–35It is necessary to study the polymer film in a solution that does not contain the monomer, so that any response of current arises solely due to the connection of Sa@AgNPs to the surface of GCE. The electrochemical response of the poly-Sa@AgNPs, formed on the GCE surface was studied in the absence of monomer in 0.1 mol L−1 acetate buffer pH 4.6 using cyclic voltammetry. The polymer film gave one oxidation peak at about +0.300 V. This peak was similar to those given by the polymerization solution. The oxidation peak is attributed to the oxidation of crocin, located in the polymer film.34,35 Furthermore, when cycling the potential with a scan rate ranging from 15 to 100 mV s−1, the resulting peak current changed linearly with the square root of the scan rate, consistent with a diffusion-controlled oxidation process.34
3.3 Effect of pH
A comprehensive pH study was conducted on three distinct solutions employed in the experimental process. Firstly, the polymerization solution utilized for depositing silver nanoparticles onto the electrode underwent rigorous investigation. Subsequent analysis revealed that the most favorable signal, following cyclic voltammetry, was achieved when employing a buffer solution of acetic acid with a pH of 5.6. At the second solution, where the interaction of amoxicillin with the modified electrode took place, a combination of 0.1 mol L−1 NaH2PO4/Na2HPO4 with a pH of 7.0, along with 0.05 mol L−1 KBr, was identified as optimal for the intended reaction. The interaction time was also scrutinized, with a stirring duration of 180 seconds proving to be the most effective. Lastly, for square wave voltammetry application, the chosen buffer consisted of 0.1 mol L−1 acetate buffer with a pH of 4.0, supplemented by 0.01 mol L−1 NaNO3. The comprehensive pH study on these solutions significantly contributed to refining the experimental conditions, thereby ensuring enhanced performance and accuracy in the electrochemical analysis of amoxicillin (Fig. 3).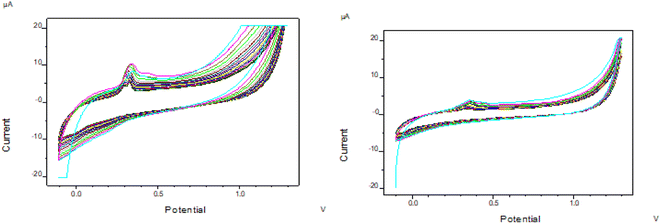 | ||
| Fig. 3 Comparison of cyclic voltammograms for AgNPs electrodeposition on the GCE at pH 5.6 (left) and pH 6.6 (right). | ||
3.4 Comparison of unmodified GCE with modified GCE
The comparison between the unmodified glassy carbon electrode (GCE) and the modified with AgNPs GCE, along with the modified GCE electrode after interaction with AMX reveals intriguing insights. Initially, the unmodified GCE electrode exhibits no discernible signal when subjected to cyclic voltammetry. However, upon introducing AgNPs as modifiers to the GCE, a distinct signal becomes evident, suggesting the enhanced electrochemical response facilitated by the presence of silver nanoparticles. Notably, the modified GCE electrode, after undergoing interaction with AMX, demonstrates a further amplification of the signal, indicating a significant augmentation in sensitivity potentially attributable to the specific AMX-AgNPs interactions. This comparative analysis underscores the substantial impact of AgNPs modification on the electrochemical behavior of the GCE electrode and underscores its potential applicability for sensitive amoxicillin detection (Fig. 4).3.5 Electrochemical behavior of amoxicillin on saffron-conjugated silver nanoparticle glassy carbon electrode
Square wave voltammetry (SWV) was used to evaluate the electrochemical characteristics of amoxicillin on GCE and poly-AgNPs@Sa-GCE in pH 4.6 acetate buffer, Fig. 5. Anodic square wave voltammograms of amoxicillin on poly-AgNPs@Sa-GCE is given in Fig. 5. Amoxicillin on modified GCE produced an oxidation peak with a low peak current at about +0.055 V, an oxidation peak at approximately +0.286 V, and an oxidation peak at +0.893 V, which can be attributed to AMX and capped AgNPs, respectively.33,34 These findings suggest that amoxicillin interacted with AgNPs@Sa-CPE. To summarize, the presence of silver nanoparticles improved the sensitivity of the detection of amoxicillin.AMX has a lot of active sites such as hydroxyl and amino groups. Thus, according to the above cyclic and square wave voltammetric results and ref. 36, the sensing mechanism of the proposed modified GCE with silver nanoparticles toward AMX could start with the interaction of AMX molecules themselves through van der Waals interactions and/or other week bonds. The remaining active sites of AMX can easily chelate with capped AgNPs. The resulting nanosilver core and the surrounding amoxicillin molecules give the oxidation signal in both CVs and SWVs (Fig. 2 and 3) at about +0.286 V and +0.893 V. Meanwhile, the electrochemical behavior of capped AgNPs with saffron, was attributed to crocin.33,34 Thus, the oxidation of crocin expresses its capacity to donate electrons accordingly to Armellini et al., 2017. Therefore, the anodic process of crocin occurs through two one-electron oxidation and one proton process processes33,34 at +0.286 V and +0.893 V (Fig. 3).
 | ||
| Fig. 5 Anodic square wave voltammograms of 0.6 ng L−1 amoxicillin on poly-AgNPs@Sa-GCE in acetate buffer pH 4.6. Experimental conditions as described in the Material and methods section. | ||
Furthermore, as can be seen in Fig. 5 the oxidation signal of the peak at +0.286 V, which was attributed to the oxidation of capped silver nanoparticles with saffron surrounded with AMX is enhanced compared to the other two oxidation peaks. Therefore, we selected to study this oxidation peak in subsequent experiments.
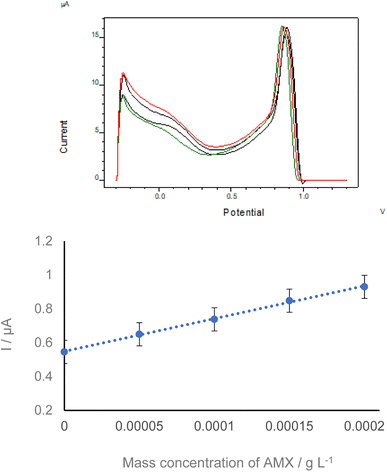
3.6 Analytical performance of the proposed sensor
The determination of AMX on AgNPs@Sa-GCE under the chosen conditions was evaluated. Fig. 6 shows the SWVs obtained after the interaction of different mass concentrations of AMX with AgNPs@Sa-GCE. Oxidation peak at +0.150 V was chosen, which was ascribed to the oxidation of AMX, in subsequent experiments. As can be seen from Fig. 6 the oxidation peak current of AMX was increased with the progressive increment of the mass concentration of AMX, indicating increased sensitivity of the proposed electrochemical sensor. The calibration curve, shown in the inset of Fig. 6, was found to be linear in the mass concentration range of AMX from 1.273 × 10−4 g L−1 to 2.217 × 10−3 g L−1, with a linear correlation coefficient of 0.9998. The linear regression equation was y(Δl/A) = 6.011 × 10−4 (±6.743 × 10−6)x + 4.827 × 10−8 (±7.649 × 10−9). The limit of detection was calculated as 3 × sb/a where sb represents the standard deviation of the ordinate on the axes origin and a is the slope of the linear portion of the calibration curve. The detection limit was 4.199 × 10−5 g L−1. The intra-day precision of the assay was adequate, with relative standard deviations of AMX at 9.199 10−5 g L−1 and 1.194 10−4 g L−1 of 4.3 and 4.0, respectively.3.7 Determination of amoxicillin in real urine sample on saffron-conjugated silver nanoparticles on a glassy carbon electrode
The suggested approach was implemented to measure the concentration of amoxicillin in a urine sample, taken from one of our lab volunteers, using the standard solution addition method. The corresponding voltammograms and the calibration curve of the addition of the standard solution to the urine sample are presented in Fig. 7. The regression equation for the urine sample (Fig. 7) was obtained to be y(A) = (1934 ± 46.60)x (g L−1) + (0.549 ± 0.006) with a linear correlation coefficient of r = 0.9991. The proposed method showed linearity in the range of amoxicillin mass concentrations from 0.00 to 2.00 × 10−4 g L−1. The detection limit was found to be 9.739 × 10−6 g L−1. The relative standard deviation between urine samples having the same amoxicillin content showed some variations in the results, which made it challenging to determine AMX concentrations precisely. However, this method could be useful in confirming the presence of AMX at mass concentrations greater than 1.273 × 10−4 g L−1 ng L−1 in aqueous samples and 2.951 × 10−5 g L−1 in urine samples. Therefore, this electrochemical sensor has the potential to be a dependable and sensitive detection tool for the determination of AMX in clinical samples.3.8 Interference study
The ability to determine AMX in the presence of other organic clinical and/or wastewater compounds, including uric acid, ascorbic acid, valine, methionine and phenylalanine were investigated. The SWV experiments were performed in the potential range of −0.300 to +1.300 V in 0.1 mol L−1 phosphate buffer at pH 7.0 containing 1.368 104 μmol L−1 of each interference, and AMX concentration of 136.8 μmol L−1. In all the SWV experiments, no overlap was observed between the AMX oxidation peak and the oxidation peaks of the interfering substances. Furthermore, the interfering substances did not significantly shift the AMX oxidation peak, indicating that the analytical signal did not suffer interference from these organic clinical and/or wastewater compounds. The results of the interference study at a mass concentration ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, are shown in Table 2.
1, are shown in Table 2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
| Interference | %R at 50 mg L−1 AMX |
|---|---|
| Ascorbic acid | 99.5% |
| Valine | 100.4% |
| Methionine | 97.9% |
| Uric acid | 103.0% |
| Phenylalanine | 97.4% |
4. Conclusion and future challenges
Overall, modified electrodes with silver nanoparticles have demonstrated great potential for the sensitive and selective determination of antibiotic of amoxicillin antibiotic in urine sample. We investigated the electrochemical behavior of a modified glassy carbon electrode using cyclic voltammetry and differential pulse voltammetry. The sensor, based on saffron-conjugated silver nanoparticles on a glassy carbon electrode, showed increased sensitivity as the concentration of AMX increased. The calibration curve was linear in the concentration range from 1.273 × 10−4 g L−1 to 2.217 × 10−3 g L−1, with a high linear correlation coefficient of 0.9998. The detection limit was determined to be 4.199 × 10−5 g L−1. The precision of the sensor was adequate, with relative standard deviations of 4.3% and 4.0% for AMX concentrations of 9.199 × 10−5 g L−1 and 1.194 × 10−4 g L−1, respectively. The proposed electrochemical sensor was then applied to determine AMX concentration in a urine sample. The method showed linearity over the amoxicillin concentration range from 0.00 to 2.00 × 10−4 g L−1, with a detection limit of 9.739 × 10−6 g L−1.Although some variation was observed in the results of urine samples with the same amoxicillin content, the method proved useful for confirming the presence of AMX at concentrations above 1.273 × 10−4 g L−1 in aqueous samples and 2.951 × 10−5 g L−1 in urine samples.
Additionally, an interference study was performed to evaluate the ability of the sensor to determine AMX in the presence of other organic clinical and/or waste compounds. The results showed that the oxidation peak of AMX does not overlap with the oxidation peaks of interfering substances such as uric acid, ascorbic acid, valine, methionine and phenylalanine. The interfering substances did not significantly affect the AMX oxidation peak, demonstrating that the analytical signal of the sensor remained unaffected by these compounds.
This field will continue to evolve as new and better methods for analyzing the surface of electrodes are developed. However, further research is needed in order to improve the stability of nanoparticle-modified electrodes. The stability of nanoparticles-modified electrodes can vary depending on the conditions of the electroanalytical measurement. Finally different strategies need to be developed to overcome interferences from other compounds in order to improve the accuracy of the measurements.
Consent
We hereby consent that:All experiments were performed in compliance with relevant laws or guidelines; all experiments followed institutional guidelines; the institutional committee(s) approved the experiments; informed consent from human subject volunteer who provided the urine sample.
Conflicts of interest
There are no conflicts to declare.References
- M. Seifrtová, L. Nováková, C. Lino, A. Pena and P. Solich, An Overview of Analytical Methodologies for the Determination of Antibiotics in Environmental Waters, Anal. Chim. Acta, 2009, 649(2), 158–179, DOI:10.1016/j.aca.2009.07.031.
- R. I. Aminov, The Role of Antibiotics and Antibiotic Resistance in Nature, Environ. Microbiol., 2009, 11(12), 2970–2988, DOI:10.1111/j.1462-2920.2009.01972.x.
- J. Anomaly, Antibiotics and Animal Agriculture: The Need for Global Collective Action, in Ethics and Drug Resistance : Collective Responsibility for Global Public Health, ed. M. J. Selgelid, and E. Jamrozik, 2020, p. 452 Search PubMed.
- L. Jie and L. Qingsong, Optimization and Application of Solid Phase Extraction-High Performance Liquid Chromatography-Tandem Mass Spectrometry for Determination of 13 Antibiotics, Environ. Chem., 2022, 41(5) DOI:10.7524/j.issn.0254-6108.2022021302.
- N. F. Semail, A. S. Abdul Keyon, B. Saad, S. Kamaruzaman, N. N. Mohamad Zain, V. Lim, M. Miskam, W. N. Wan Abdullah, N. Yahaya and D. D. Y. Chen, Simultaneous Preconcentration and Determination of Sulfonamide Antibiotics in Milk and Yoghurt by Dynamic PH Junction Focusing Coupled with Capillary Electrophoresis, Talanta, 2022, 236, 122833, DOI:10.1016/j.talanta.2021.122833.
- M. Senthilkumar, N. Amaresan, and A. Sankaranarayanan, Detection of Pyoluteorin by Thin Layer Chromatography, Plant-Microbe Interactions, Springer, 2021, DOI:10.1007/978-1-0716-1080-0_28.
- D. Barzallo, E. Palacio, J. March and L. Ferrer, 3D Printed Device Coated with Solid-Phase Extraction Resin for the on-Site Extraction of Seven Sulfonamides from Environmental Water Samples Preceding HPLC-DAD Analysis, Environ. Anal. Chem., 2022, 25, 108609 Search PubMed.
- F. Shen, Y. J. Xu, Y. Wang, J. Chen and S. Wang, Rapid and Ultra-Trace Levels Analysis of 33 Antibiotics in Water by on-Line Solid-Phase Extraction with Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry, J. Chromatogr. A, 2022, 1677, 463304, DOI:10.1016/j.chroma.2022.463304.
- S. A. Khatibi, S. Hamidi and M. R. Siahi-Shadbad, Application of Liquid-Liquid Extraction for the Determination of Antibiotics in the Foodstuff: Recent Trends and Developments, Crit. Rev. Anal. Chem., 2022, 52(2), 327–342, DOI:10.1080/10408347.2020.1798211.
- T. T. T. Tran, M. N. Do, T. N. H. Dang, Q. H. Tran, V. T. Le, A. Q. Dao and Y. Vasseghian, A State-of-the-Art Review on Graphene-Based Nanomaterials to Determine Antibiotics by Electrochemical Techniques, Environ. Res., 2022, 208(December 2021), 112744, DOI:10.1016/j.envres.2022.112744.
- E. M. Materón, A. Wong, T. A. Freitas, R. C. Faria and O. N. Oliveira, A Sensitive Electrochemical Detection of Metronidazole in Synthetic Serum and Urine Samples Using Low-Cost Screen-Printed Electrodes Modified with Reduced Graphene Oxide and C60, J. Pharm. Anal., 2021, 11(5), 646–652, DOI:10.1016/j.jpha.2021.03.004.
- J. A. Cox, M. E. Tess and T. E. Cummings, Electroanalytical Methods Based on Modified Electrodes: A Review of Recent Advances, Rev. Anal. Chem., 1996, 15(3), 173–223, DOI:10.1515/REVAC.1996.15.3.173.
- I. Cesarino, V. Cesarino and M. R. V. Lanza, Carbon Nanotubes Modified with Antimony Nanoparticles in a Paraffin Composite Electrode: Simultaneous Determination of Sulfamethoxazole and Trimethoprim, Sens. Actuators, B, 2013, 188, 1293–1299, DOI:10.1016/j.snb.2013.08.047.
- A. Feizollahi, A. A. Rafati, P. Assari and R. Asadpour Joghani, Development of an Electrochemical Sensor for the Determination of Antibiotic Sulfamethazine in Cow Milk Using Graphene Oxide Decorated with Cu-Ag Core-Shell Nanoparticles, Anal. Methods, 2021, 13(7), 910–917, 10.1039/d0ay02261f.
- T. Kokulnathan, T. S. K. Sharma, S. M. Chen, T. W. Chen and B. Dinesh, Ex-Situ Decoration of Graphene Oxide with Palladium Nanoparticles for the Highly Sensitive and Selective Electrochemical Determination of Chloramphenicol in Food and Biological Samples, J. Taiwan Inst. Chem. Eng., 2018, 89, 26–38, DOI:10.1016/j.jtice.2018.04.030.
- S. A. R. Alavi-Tabari, M. A. Khalilzadeh and H. Karimi-Maleh, Simultaneous Determination of Doxorubicin and Dasatinib as Two Breast Anticancer Drugs Uses an Amplified Sensor with Ionic Liquid and ZnO Nanoparticle, J. Electroanal. Chem., 2018, 811, 84–88, DOI:10.1016/j.jelechem.2018.01.034.
- N. B. Simioni, T. A. Silva, G. G. Oliveira and O. Fatibello-Filho, A Nanodiamond-Based Electrochemical Sensor for the Determination of Pyrazinamide Antibiotic, Sens. Actuators, B, 2017, 250, 315–323, DOI:10.1016/j.snb.2017.04.175.
- K. Ghanbari and M. Roushani, A Novel Electrochemical Aptasensor for Highly Sensitive and Quantitative Detection of the Streptomycin Antibiotic, Bioelectrochemistry, 2018, 120, 43–48, DOI:10.1016/j.bioelechem.2017.11.006.
- M. Nosuhi and A. Nezamzadeh-Ejhieh, Comprehensive Study on the Electrocatalytic Effect of Copper – Doped Nano-Clinoptilolite towards Amoxicillin at the Modified Carbon Paste Electrode – Solution Interface, J. Colloid Interface Sci., 2017, 497, 66–72, DOI:10.1016/j.jcis.2017.02.055.
- A. Afkhami, F. Soltani-Felehgari and T. Madrakian, Gold Nanoparticles Modified Carbon Paste Electrode as an Efficient Electrochemical Sensor for Rapid and Sensitive Determination of Cefixime in Urine and Pharmaceutical Samples, Electrochim. Acta, 2013, 103, 125–133, DOI:10.1016/j.electacta.2013.04.064.
- P. Manjunatha and Y. A. Nayaka, Cetyltrimethylammonium Bromide-Gold Nanoparticles Composite Modified Pencil Graphite Electrode for the Electrochemical Investigation of Cefixime Present in Pharmaceutical Formulations and Biology, Chem. Data Collect., 2019, 21, 100217, DOI:10.1016/j.cdc.2019.100217.
- N. Kumar, Rosy and R. N. Goyal, Gold-Palladium Nanoparticles Aided Electrochemically Reduced Graphene Oxide Sensor for the Simultaneous Estimation of Lomefloxacin and Amoxicillin, Sens. Actuators, B, 2017, 243, 658–668, DOI:10.1016/j.snb.2016.12.025.
- H. Zhai, Z. Liang, Z. Chen, H. Wang, Z. Liu, Z. Su and Q. Zhou, Simultaneous Detection of Metronidazole and Chloramphenicol by Differential Pulse Stripping Voltammetry Using a Silver Nanoparticles/Sulfonate Functionalized Graphene Modified Glassy Carbon Electrode, Electrochim. Acta, 2015, 171, 105–113, DOI:10.1016/j.electacta.2015.03.140.
- S. Cheng, H. Liu, H. Zhang, G. Chu, Y. Guo and X. Sun, Ultrasensitive Electrochemiluminescence Aptasensor for Kanamycin Detection Based on Silver Nanoparticle-Catalyzed Chemiluminescent Reaction between Luminol and Hydrogen Peroxide, Sens. Actuators, B, 2020, 304, 127367, DOI:10.1016/j.snb.2019.127367.
- S. Atif, J. A. Baig, H. I. Afridi, T. G. Kazi and M. Waris, Novel Nontoxic Electrochemical Method for the Detection of Cefadroxil in Pharmaceutical Formulations and Biological Samples, Microchem. J., 2020, 154(December 2019), 104574, DOI:10.1016/j.microc.2019.104574.
- S. Liu, G. Lai, H. Zhang and A. Yu, Amperometric Aptasensing of Chloramphenicol at a Glassy Carbon Electrode Modified with a Nanocomposite Consisting of Graphene and Silver Nanoparticles, Microchim. Acta, 2017, 184(5), 1445–1451, DOI:10.1007/s00604-017-2138-y.
- R. T. Kushikawa, M. R. Silva, A. C. D. Angelo and M. F. S. Teixeira, Construction of an Electrochemical Sensing Platform Based on Platinum Nanoparticles Supported on Carbon for Tetracycline Determination, Sens. Actuators, B, 2016, 228, 207–213, DOI:10.1016/j.snb.2016.01.009.
- H. Karimi-Maleh, F. Tahernejad-Javazmi, V. K. Gupta, H. Ahmar and M. H. Asadi, A Novel Biosensor for Liquid Phase Determination of Glutathione and Amoxicillin in Biological and Pharmaceutical Samples Using a ZnO/CNTs Nanocomposite/Catechol Derivative Modified Electrode, J. Mol. Liq., 2014, 196, 258–263, DOI:10.1016/j.molliq.2014.03.049.
- V. Velusamy, S. Palanisamy, T. Kokulnathan, S. W. Chen, T. C. K. Yang, C. E. Banks and S. K. Pramanik, Novel Electrochemical Synthesis of Copper Oxide Nanoparticles Decorated Graphene-β-Cyclodextrin Composite for Trace-Level Detection of Antibiotic Drug Metronidazole, J. Colloid Interface Sci., 2018, 530, 37–45, DOI:10.1016/j.jcis.2018.06.056.
- C. Buzea, I. I. Pacheco and K. Robbie, Nanomaterials and Nanoparticles: Sources and Toxicity, Biointerphases, 2019, 71 DOI:10.1116/1.2815690.
- Y. A. Krutyakov, A. A. Kudrinskiy, A. Y. Olenin and G. V. Lisichkin, Synthesis and Properties of Silver Nanoparticles Advances and Prospects, Russ. Chem. Rev., 2008, 77(3) DOI:10.1070/RC2008v077n03ABEH003751.
- S. Zeng, D. Baillargeat, H. Ho and K. Yong, Nanomaterials Enhanced Surface Plasmon Resonance for Biological and Chemical Sensing Applications, Chem. Soc. Rev., 2014, 43, 3426–3452, 10.1039/c3cs60479a.
- S. Karastogianni, I. Paraschi and S. Girousi, Electrochemical sensing of the maple syrup urine disease biomarker valine, using saffron-silver nanoparticles, Biosens. Bioelectron.: X, 2022, 12, 100275, DOI:10.1016/j.biosx.2022.100275.
- R. Armellini, D. Compagnone, M. Scampicchio and P. Pittia, Hydrogen and Atom Transfer Activity of Saffron Extracts by Square Wave Voltammetry, Electroanalysis, 2017, 29, 521–528 CrossRef CAS.
- R. A. Dar, P. K. Brahman, N. Khurana, J. A. Wagay, Z. A. Lone, M. A. Ganaie and K. S. Pitre, Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays, Arabian J. Chem., 2017, 10, S1119–S1128 CrossRef CAS.
- L. Ping, L. Juan, W. Changzhu, W. Qingsheng and L. Jian, Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles, Nanotechnology, 2005, 16, 1912–1917 CrossRef.
- M. Zhu, R. Li, M. Lai, H. Ye, N. Long, J. Ye and J. Wang, Copper nanoparticles incorporating a cationic surfactant-graphene modified carbon paste electrode for the simultaneous determination of gatifloxacin and pefloxacin, J. Electroanal. Chem., 2020, 857, 113730, DOI:10.1016/j.jelechem.2019.113730.
- X. Dai, G. Wildgoose, C. Salter, A. Crossley and R. Compton, Electroanalysis using macro-, micro-, and nanochemical architectures on electrode surfaces. Bulk surface modification of glassy carbon microspheres with gold nanoparticles and their electrical wiring using carbon nanotubes, Anal. Chem., 2006, 78(17), 6102–6108 CrossRef CAS PubMed.
- H. Wang, H. Zhao, X. Quan and S. Chen, Electrochemical Determination of Tetracycline Using Molecularly Imprinted Polymer Modified Carbon Nanotube-Gold Nanoparticles Electrode, Electroanalysis, 2011, 23(8), 1863–1869 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |