Open Access Article
Open Access ArticleCalibration and validation of ultraviolet time-of-flight mass spectrometry for online measurement of exhaled ciprofol
Xiaoxiao
Li†
ab,
Pan
Chang†
ab,
Xing
Liu
ab,
Zhongjun
Zhao
 c,
Wenwen
Li
c,
Yi
Kang
ab,
Yixiang
Duan
c and
Wensheng
Zhang
c,
Wenwen
Li
c,
Yi
Kang
ab,
Yixiang
Duan
c and
Wensheng
Zhang
 *ab
*ab
aDepartment of Anaesthesiology, West China Hospital, Sichuan University, China. E-mail: zhang_ws@scu.edu.cn
bLaboratory of Anaesthesia and Critical Care Medicine, National-Local Joint Engineering Research Centre of Translational Medicine of Anaesthesiology, West China Hospital, Sichuan University, China
cSchool of Mechanical Engineering, Sichuan University, China
First published on 5th August 2023
Abstract
Ciprofol (HSK 3486, C14H20O), a novel 2,6-disubstituted phenol derivative similar to propofol, is a new type of intravenous general anaesthetic. We found that the exhaled ciprofol concentration could be measured online by ultraviolet time-of-flight mass spectrometry (UV-TOFMS), which could be used to predict the plasma concentration and anaesthetic effects of ciprofol. In this study, we present the calibration method and validation results of UV-TOFMS for the quantification of ciprofol gas. Using a self-developed gas generator to prepare different concentrations of ciprofol calibration gas, we found a linear correlation between the concentration and intensity of ciprofol from 0 parts per trillion by level (pptv) to 485.85 pptv (R2 = 0.9987). The limit of quantification was 48.59 pptv and the limit of detection was 7.83 pptv. The imprecision was 12.44% at 97.17 pptv and was 8.96% at 485.85 pptv. The carry-over duration was 120 seconds. In addition, we performed a continuous infusion of ciprofol in beagles, measured the exhaled concentration of ciprofol by UV-TOFMS, determined the plasma concentration by high-performance liquid chromatography, and monitored the anaesthetic effects as reflected by the bispectral index value. The results showed that the exhaled and plasma concentrations of ciprofol were linearly correlated. The exhaled ciprofol concentration correlated well with the anaesthetic effect. The study showed that we could use UV-TOFMS to provide a continuous measurement of gaseous ciprofol concentration at 20 second intervals.
1 Introduction
Ciprofol (HSK 3486, C14H20O), a novel 2,6-disubstituted phenol derivative similar to propofol, has been approved by the National Medical Products Administration of China and has undergone phase III clinical trials in the United States.1,2 Ciprofol's anaesthetic effects, onset, and recovery time were similar to those of propofol, but the injection pain was significantly reduced.3–5 The concentration of ciprofol should be timely detected as too high or too low concentrations may increase the incidence of adverse effects including hypotension, bradycardia, respiratory depression, and intraoperative awareness.5,6Like other intravenous anaesthetics, ciprofol is administered primarily on a dose–response basis.7 Due to differences in drug distribution and metabolism, drug concentrations in the blood and at the site of action are highly variable and difficult to predict from dose and patient characteristics.8 Overdosing or underdosing of drugs is common in clinical practice. Real-time monitoring of ciprofol for titration to the appropriate dose is essential. However, no studies on real-time monitoring of ciprofol concentration are currently available.
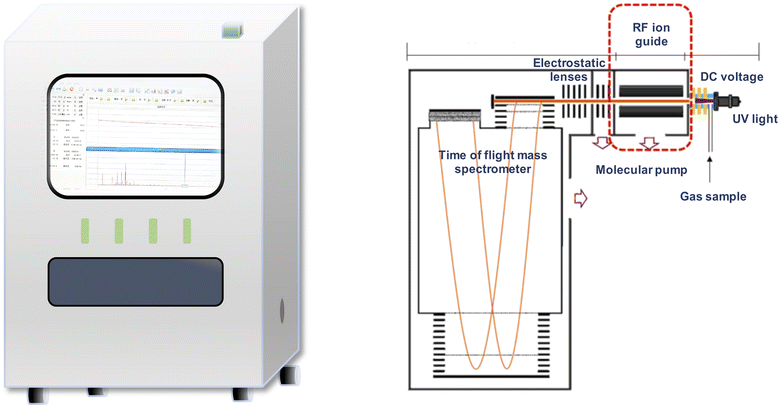
Our team has developed a type of on-line spectrometer that combines ultraviolet (UV) ionization with time-of-flight mass spectrometry (TOFMS) to analyze the concentration of ciprofol in exhaled breath in real time.9 Calibration is required prior to using this instrument to quantify ciprofol. Therefore, this study was designed to calibrate and analytically validate a UV-TOFMS system for on-line measurement of exhaled ciprofol.
2 Materials and methods
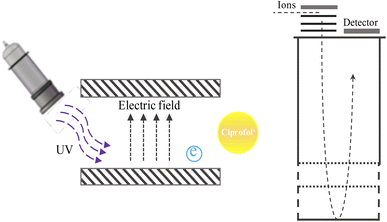
2.1. UV-TOFMS
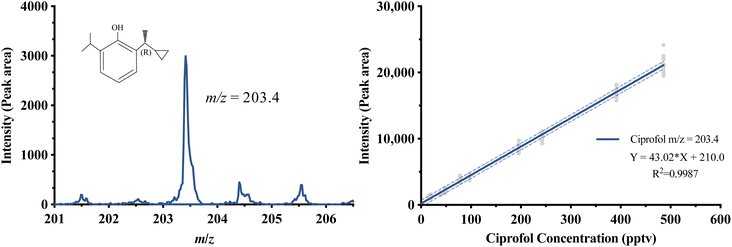
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (i.e., measurement period is 20 seconds per time); pulse interval 50. An m/z of 203.4 in the TOFMS spectrum was selected as the mass peak of ciprofol (i.e. the dehydrogenated molecular ion peak), which was obtained by testing ciprofol standards using UV-TOFMS.
000 (i.e., measurement period is 20 seconds per time); pulse interval 50. An m/z of 203.4 in the TOFMS spectrum was selected as the mass peak of ciprofol (i.e. the dehydrogenated molecular ion peak), which was obtained by testing ciprofol standards using UV-TOFMS.
2.2. Calibration
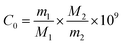 | (1) |
 | (2) |
By replacing the ciprofol solution (C2–C6, flow rate 0.8 g h−1) and changing the carrier gas flow rate (N2, flow rate 600 ml h−1, 800 ml h−1), different concentrations of ciprofol calibration gas (0 parts per trillion by level (pptv), 15.66 pptv, 19.43 pptv, 39.15 pptv, 48.59 pptv, 78.30 pptv, 97.17 pptv, 195.74 pptv, 242.93 pptv, 391.48 pptv, and 485.85 pptv) can be obtained from the metal evaporator (150 °C). Then the ciprofol calibration gas is directly introduced into the UV-TOFMS. Each concentration was held for 10 min, corresponding to 30 measurements. Before and after each concentration, 10 blank measurements were obtained.
2.3. Validation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the LOD and 10
1 for the LOD and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the LOQ. Specifically, the test concentration was gradually reduced by 0.1 ml from the ciprofol concentration (C1) solution (i.e., 0.3 ml + 49.7 ml, 0.2 ml + 49.8 ml, 0.1 ml + 49.9 ml). Each concentration was maintained for 10 minutes. The noise was calculated as the average intensity of 10 initial blank measurements.
1 for the LOQ. Specifically, the test concentration was gradually reduced by 0.1 ml from the ciprofol concentration (C1) solution (i.e., 0.3 ml + 49.7 ml, 0.2 ml + 49.8 ml, 0.1 ml + 49.9 ml). Each concentration was maintained for 10 minutes. The noise was calculated as the average intensity of 10 initial blank measurements.
2.4. Animal experiments
This study was approved by the Animal Ethics Committee of West China Hospital, Sichuan University (20220420001). Five male beagles (12–18 months, 9–14 kg, Chengdu DOSSY Experimental Animals Co., Ltd.) were involved in this study. Bilateral forearm veins were punctured and catheterized (one side for drug administration and the other for blood sampling). The interval of blood sampling was 10 min. Then, the bispectral index (BIS, an indicator of the anaesthetic effect) electrodes were then placed on the beagles' foreheads to monitor the depth of anaesthesia, and the data acquisition interval was 20 seconds. The anaesthetic tiletamine hydrochloride and zolazepam hydrochloride for injection (Zoletil® 50, Virbac Group, France) was injected intravenously at a dose of 5 mg kg−1 for anaesthesia induction. After the loss of the righting reflex, a tracheal tube (ID: #7.0; made of polyvinyl chloride; Tuoren, Zhengzhou, China) was intubated under a laryngoscope and connected to a ventilator (Boaray 700, Probe Company, Shenzhen, China), the parameters of which were set as follows: volume control ventilation mode, pure oxygen supply, a flow rate of 2 L min−1, a respiratory rate of 20 times per min, and a tidal volume of 10 ml kg−1. The dosage of ciprofol administered was 0.125 mg kg−1 min−1 (dose that eliminated the righting reflex in 95% of beagles) for up to 1 hour (BeneFusion n series, Shenzhen Mindray, Shenzhen, China). During the whole procedure, the body temperature of beagles was maintained at 36.5 ± 0.5 °C. Upon awakening, the tracheal tube and the intravenous needle cannula were removed. After full recovery, the beagles were returned to the animal room.A three-way valve (made of polycarbonate) was used to connect the tracheal tube of the breathing circuit (made of polytetrafluoroethylene) to the UV-TOFMS. Detection conditions were set as shown above. Ciprofol was continuously detected at 20 seconds per time. An m/z of 203.4 in the TOFMS spectrum was selected as the mass peak of ciprofol. The ciprofol concentration in exhaled air was calculated according to the calibration curve.
2.5. Statistical analysis
Data analysis for the TOFMS spectra of ciprofol and peak intensities was performed by the built-in calculation software. Statistical analyses were performed with Graphpad Prism (version 9.5, Graphpad Prism Software, USA). The Shapiro–Wilk test was used to assess the normality of data. One-way ANOVA was used for normally distributed data. Alternatively, the one-way ANOVA on rank was conducted. P < 0.05 was considered significant.3 Results
3.1. The linear calibration curves
Fig. 4 shows the calibration curve of ciprofol signal intensity against gas concentration in UV-TOFMS. The middle 20 (of 30) values of each concentration were evaluated (gray dots). The linear calibration curve (blue line) shows a coefficient of determination of 0.9987 (y = 43.02x + 210.0) between 0 pptv and 485.85 pptv.3.2. LOD and LOQ
Based on the signal-to-noise ratio, the LOD was 7.83 pptv (average peak intensity: 668) and the LOQ was 48.59 pptv (average peak intensity: 2056).3.3. Precision
The precision measurements at 97.17 pptv and 485.85 pptv had a relative standard deviation of 6.53% and 4.40% and a statistical range of 12.44% and 8.96% of the mean.3.4. Carry over
Concentrations of 97.17 pptv and 485.85 pptv were maintained for 1 h before changing the concentration to 0 pptv. The first value after the ciprofol evaporation stopped shows a carry-over of 3.2% and 5.9%, respectively, of the average signal intensity at 97.17 pptv and 485.85 pptv.3.5. Animal experiments
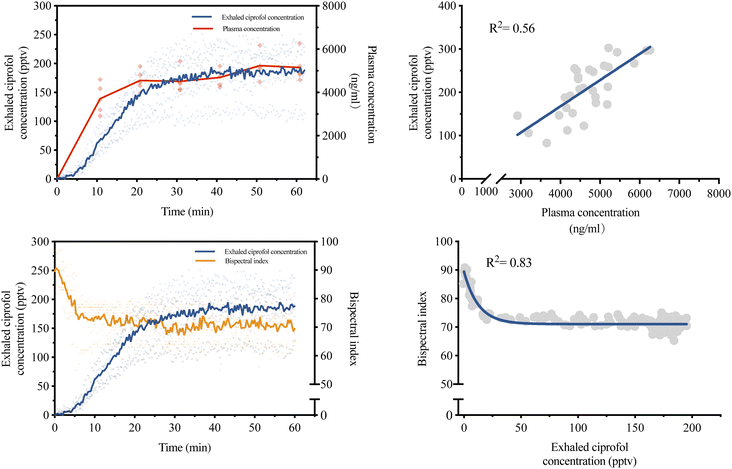
A total of 5 beagles were involved in this experiment, with an age of 12.89 ± 1.55 month and weight of 11.83 ± 1.13 kg. All beagles survived and were returned to the animal room after full awakening. The administration dosage was 91.50 ± 1.62 mg. The results showed that ciprofol concentrations in exhaled breath and plasma were linearly correlated (y = 0.06058x − 74.75, R2 = 0.56), and the correlation of exhaled ciprofol concentration and BIS can be well fitted with the one-phase exponential decay model (y = 18.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−0.07888x) + 89.44, R2 = 0.83) (Fig. 5).
exp(−0.07888x) + 89.44, R2 = 0.83) (Fig. 5).
4 Discussion
Online monitoring of the concentrations of anaesthetics is a major problem in clinical anaesthesia and one of the important directions for future anaesthesia research. The only intravenous anaesthetic that can be monitored online in published studies is propofol. Our study is the first to achieve online monitoring of anaesthetics other than propofol.Current techniques for on-line monitoring of propofol include proton transfer reaction mass spectrometry (PTR-MS),11 selected ion flow tube mass spectrometry (SIFT-MS),12 ion molecular reaction mass spectrometry (IMR-MS),13 gas chromatography combined with a surface acoustic wave sensor (GC-SAW),14 and ion mobility spectrometry (IMS).15 The first three are reported to be not suitable in the operating room due to their large instrument size, loud operating noise and complex operation.16 In studies using GC-SAW, exhaled breath was collected offline, which may have resulted in the loss of important drug concentration information.17 The performance of IMS is easily disturbed by humidity, which is high in exhaled breath.18 Using UV-TOFMS, gas samples can be analysed online without pre-separation. The sensitivity of UV-TOFMS can reach the pptv level and it is insensitive to humidity, which meets the requirements for the detection of trace volatile organic compounds in exhaled breath.
In the pilot trials, we have detected the presence of ciprofol in exhaled breath of rats and beagles using the UV-TOFMS instrument and found that the levels of ciprofol in exhaled breath were under 400 pptv. Therefore, to validate this calibration method, we prepared the target standard gas concentration in the range of 0 pptv to 485.85 pptv in this study. The range of ciprofol concentrations in human exhaled breath is unknown because we have not yet used the device on humans to measure ciprofol concentrations. However, ciprofol is a reconstituted drug of propofol with relatively similar physicochemical properties.3 Combined with the distribution of data from our completed UV-TOFMS-based studies on propofol in rats, beagles, and humans, we believe that the range of ciprofol concentrations in beagle exhaled breath at equivalent doses is similar to that in humans. Therefore, the concentration ranges investigated in this study are suitable for future extension to humans. The reason why the concentration of ciprofol in expired air is so low, in our opinion, is related to the saturated vapor pressure of ciprofol.16 The volatility of a substance is related to its saturated vapor pressure, which is lower for propofol.19 Like propofol, ciprofol is more lipid soluble, and less volatile gas is formed from free ciprofol in the blood and passes through the alveolar membrane.20 Our study is the first to report the presence of ciprofol in exhaled breath. There are no other studies available for comparison.
The low concentrations of ciprofol can be well measured using UV-TOFMS, which is a mass spectrometer based on UV recombination ionization and time-of-flight mass spectrometry technology. With the soft ionization of UV, pre-separation of air samples is unnecessary, and most volatile organic compounds in air samples can be ionized immediately. Unlike electron ionization, UV ionization can avoid the production of fragment ions, allowing for the direct detection of prototypes.21 In addition, about 23 ml of air sample is directly delivered into the UV-TOFMS under constant pressure without any other processing. This instrument did not extract more gas compared to other instruments.22 In addition, the instrument adopts multi-stage vacuum with high-efficiency ion transfer design, which greatly improves the performance of the instrument. When combined with TOFMS, this instrument has the capability for performing online analysis with high sensitivity up to the pptv level. The response time of the instrument is less than 0.5 seconds. Volatile organic compounds in air samples can be ionized instantaneously and simultaneously, and the results of all component concentrations can be calculated in real time by the built-in software, allowing dynamic data acquisition. However, it should be mentioned that the size of the current prototype device is not too small, and requires a certain space in the operating room. The internal vacuum molecular pump takes up a larger space. In the future, the size of the pump can be reduced accordingly. This will reduce the volume of the entire machine.
Ciprofol is modified from propofol, so it has similar physical and chemical properties to those of propofol, i.e. it also has high viscosity, which places higher demands on the tubing material in the calibration system.21 We used an inert material PEEK tube for the introduction of ciprofol into the instrument and wrapped it with sponge. The temperature was controlled at 100 °C by using a temperature control switch. The temperature-controlled sampling tube can effectively avoid the residue of ciprofol gas.23 It can also speed up the response process of the instrument. In the calibration system, PEEK tubing or an inert metal cavity was also selected as the connection for the piping through which ciprofol passed. This was done to minimize the loss caused by the transfer process.
Unlike previous studies, we did not use commercial off-the-shelf gas generators to produce standard gas.24 This is because the finished gas generator often has a certain volume. It is difficult to reprocess and fuse it into the instrument. We hope that when the UV-TOFMS instrument is used for clinical testing in the future, the calibration system can be equipped inside the instrument, and the mass flow meter based on the scattering can be freely assembled. Therefore, we built a gas generator ourselves using two precision mass flow meters. The calibration and validation of UV-TOFMS is based on measurements of defined ciprofol gas concentrations. In this method, the gas generator can prepare the target standard gas concentration by changing the initial solution concentration of ciprofol and the carrier gas in combination with the ideal gas equation. The advantage is that the preparation method is simple and convenient, and is also more accurate than the gas dilution method using Tedlar bags.17 The disadvantage is that the generator uses the ideal gas law to estimate the concentration, which is a general method but not completely accurate.25 However, based on previous studies, it can be assumed that the associated bias is negligible. Meanwhile, the concentration of integer multiples cannot be obtained. In addition, we chose to set the evaporation tank temperature at 150 °C. The gasification temperature of propofol reported in previous studies was 40 °C or 100 °C, which leaves the possibility of incomplete gasification.24,26–28 Therefore, in this study, the temperature in the evaporation tank was set at 150 °C to completely gasify the ciprofol solution.
A LOD of 7.83 pptv and a LOQ of 48.59 pptv were obtained with a goodness of fit of 0.9987. This linearity range is well suited for calibration of ciprofol gas concentrations in rats and beagles, and sufficient to quantify ciprofol over the entire clinical range. We used as many as 11 points instead of the traditional 5 points when drawing the calibration curve. We hoped that adding more points would make the standard curves more accurate, since this was an exploratory study. Based on the exploration of good linearity, we plan to reduce the number of calibration points in the next step. Our ultimate goal is to reduce the cost and time required for gas calibration by integrating the calibration system into the UV-TOFMS and controlling the variation of the carrier gas flow rate only, without changing the concentration of the ciprofol solution. Within a good linear concentration range, it is possible to produce standard gases with different concentrations at two or three points, which would make calibration of the system easy and quick. Regarding accuracy, our data shows low variation, less than 15%. A variation of up to 15% is generally considered satisfactory from an analytical point of view.22 In this system, we used constant pressure injection to ensure the measurement accuracy of the instrument in different detection periods to a certain extent. However, the effect of 12.44% breath inaccuracy on blood concentration estimation is unclear. A carry-over effect was observed in the first 20 s after a concentration change, with a value of less than 6% of the average signal intensity for 97.17 pptv and 485.85 pptv. One reason for this effect may be the adhesion of ciprofol to the internal surfaces of the gas generator and the UV-TOFMS.29–31 All of these surfaces have the potential to contribute to carry over. The interaction of gaseous ciprofol with plastic and stainless-steel surfaces should be further investigated. However, after six measurements at 20 s intervals, the carry-over was only 2.2% at 0 pptv, which is less than the imprecision of our method. Furthermore, the low carry-over after only one minute of washout is suitable for clinical use.
From data on continuous infusion of ciprofol in beagles, we found a moderate correlation between the exhaled concentration and the plasma concentration. This may be due to the small number of subjects. However, as an exploratory experiment, this suggests that the exhaled concentration of ciprofol has the potential to predict the plasma concentration. However, as with propofol, there appears to be a significant delay in the exhaled concentration of ciprofol compared to the plasma concentration.19,31 In fact, when the corresponding standard gas in the gas generator is connected to the UV-TOFMS, the instrument can detect the presence of ciprofol in the next moment, indicating that these delays are not caused by the adsorption of the tubing, but rather by the enormous blood gas distribution coefficient of ciprofol. Achieving equilibrium between the free ciprofol in the blood and the volatile ciprofol gas takes a long time. This delay can be also exacerbated by blockage of the alveolar membrane.32 From another perspective, there is a certain delay between plasma drug concentrations and anaesthetic effects.33–35 Therefore, we further investigated whether exhaled ciprofol concentrations correlated well with anaesthetic effects. We found that the relationship between exhaled ciprofol concentration and BIS could be well fitted by the one-phase exponential decay model (R2 = 0.83), indicating that exhaled ciprofol monitoring can be used to guide the titration of depth of anaesthesia, which is more helpful to anaesthetists.
In this study, we developed and validated a method based on UV-TOFMS for the quantitative detection of ciprofol gas. In fact, this method including the UV-TOFMS instrument, the gas generator, and the gas dilution method is not only suitable for ciprofol, but also for propofol. It is inferred that propofol and ciprofol have similar structures and physicochemical properties. We have completed a series of experiments using the same calibration method for the quantitative detection of propofol gas and also obtained good results. Meanwhile, we are actively investigating the feasibility of this calibration method for other anaesthetics, such as etomidate, another liposoluble anaesthetic commonly used in clinical practice. Of course, this method also has some limitations. Firstly, the humidity of the standard gases obtained by this method is around 100%. However, the amount of water in exhaled breath is not constant during the whole surgery and the humidity may change over time. Therefore, this method needs to be further improved in future studies to achieve the purpose of obtaining gases with different humidities. Secondly, in clinical practice, anaesthesia is often the result of a combination of different anaesthetic agents, including sedatives (e.g. ciprofol and propofol), analgesics and muscle relaxants, rather than a single drug. Whether the calibration system and method can be used for the simultaneous quantitative detection of other intravenous anaesthetics requires further investigation.
5 Conclusions
The calibration and validation procedure of a UV-TOFMS with a self-developed gas generator was successfully established. The results showed that we could use the UV-TOFMS to provide continuous measurement of gaseous ciprofol concentration at 20 second intervals.Author contributions
Funding acquisition and project administration: ZWS; supervision: DYX; conceptualization: ZWS, LXX, and CP; methodology and resources: ZZJ, LWW, and DYX; investigation and validation: LXX, CP, and LX; data curation and software: LWW and LXX; formal analysis: LXX, CP, and KY; visualization and writing – original draft: LXX and CP; writing – review & editing: ZWS, LXX, CP, ZZJ, and DYX. All authors approved the submitted version of the manuscript and agreed to be personally accountable for their own contributions and to ensure that questions related to the accuracy and integrity of the work are resolved and documented.Conflicts of interest
The authors declare no conflict of interest for this work.Acknowledgements
This study was supported by the Sichuan Province Science and Technology Support Program (2023YFS0136). The authors thank Yanting Yang for her guidance and inspiration in the writing of the article.References
- NCT05478174, Efficacy and Safety of HSK3486 Compared to Propofol for Adults Undergoing Elective Surgery with General Anaesthesia, https://www.clinicaltrials.gov/, 2023-02-22 Search PubMed.
- P. Liang, M. Dai and X. Wang,
et al., Efficacy and safety of ciprofol vs. propofol for the induction and maintenance of general anaesthesia: a multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial, Eur. J. Anaesthesiol., 2023, 40, 399–406 CrossRef CAS PubMed
.
- J. Liao, M. Li and C. Huang,
et al., Pharmacodynamics and Pharmacokinetics of HSK3486, a Novel 2,6-Disubstituted Phenol Derivative as a General Anaesthetic, Front. Pharmacol., 2022, 13, 830791 CrossRef CAS PubMed
.
- Y. Teng, M. Ou and X. Wang,
et al., Efficacy and safety of ciprofol for the sedation/anaesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials, Eur. J. Pharm. Sci., 2021, 164, 105904 CrossRef CAS PubMed
.
- Z. Luo, H. Tu and X. Zhang,
et al., Efficacy and Safety of HSK3486 for Anaesthesia/Sedation in Patients Undergoing Fiberoptic Bronchoscopy: A Multicenter, Double-Blind, Propofol-Controlled, Randomized, Phase 3 Study, CNS Drugs, 2022, 36, 301–313 CrossRef CAS PubMed
.
- J. Li, X. Wang and J. Liu,
et al., Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial, Basic Clin. Pharmacol. Toxicol., 2022, 131, 138–148 CrossRef CAS PubMed
.
- B. Meibohm and H. Derendorf, Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling, Int. J. Clin. Pharmacol. Ther., 1997, 35, 401–413 CAS
.
- M. M. Struys, M. Sahinovic and B. J. Lichtenbelt,
et al., Optimizing intravenous drug administration by applying pharmacokinetic/pharmacodynamic concepts, Br. J. Anaesth., 2011, 107, 38–47 CrossRef CAS PubMed
.
-
Y. X. Duan, Z. J. Zhao and Y. T. Yang, A high-sensitivity UV-ionization time-of-flight mass spectrometer and ion time-of-flight measurement method, China Pat., CN111613514A, 2020.09.01 Search PubMed
.
- ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology, Int. Conf. Harmon., 2005, 1994, p. 17 Search PubMed.
- G. R. Harrison, A. D. Critchley, C. A. Mayhew and J. M. Thompson, Real-time breath monitoring of propofol and its volatile metabolites during surgery using a novel mass spectrometric technique: a feasibility study, Br. J. Anaesth., 2003, 91, 797–799 CrossRef CAS PubMed
.
- P. R. Boshier, J. R. Cushnir and V. Mistry,
et al., On-line, real time monitoring of exhaled trace gases by SIFT-MS in the perioperative setting: a feasibility study, Analyst, 2011, 136, 3233–3237 RSC
.
- M. Grossherr, B. Varadarajan and L. Dibbelt,
et al., Time course of ethanol and propofol exhalation after bolus injection using ion molecule reaction-mass spectrometry, Anal. Bioanal. Chem., 2011, 401, 2063–2067 CrossRef CAS PubMed
.
- H. Dong, F. Zhang and J. Chen,
et al., Evaluating Propofol Concentration in Blood From Exhaled Gas Using a Breathing-Related Partition Coefficient, Anesth. Analg., 2020, 130, 958–966 CrossRef CAS PubMed
.
- Y. Liu, Y. Gong and C. Wang,
et al., Online breath analysis of propofol during anesthesia: clinical application of membrane inlet-ion mobility spectrometry, Acta Anaesthesiol. Scand., 2015, 59, 319–328 CrossRef CAS PubMed
.
- L. M. Wirtz, S. Kreuer, T. Volk and T. Hüppe, Modern breath analysis, Med. Klin. - Intensivmed. Notfallmedizin, 2019, 114, 655–660 CrossRef CAS PubMed
.
- X. Chen, X. L. Zhang and L. Liu,
et al., Gas chromatograph-surface acoustic wave for quick real-time assessment of blood/exhaled gas ratio of propofol in humans, Br. J. Anaesth., 2014, 113, 807–814 CrossRef CAS PubMed
.
- T. Teucke, F. Maurer, L. M. Müller-Wirtz, T. Volk, D. I. Sessler and S. Kreuer, Humidity and measurement of volatile propofol using MCC-IMS (EDMON), J. Clin. Monit. Comput., 2023, 37, 493–500 CrossRef PubMed
.
- C. Hornuss, S. Praun and J. Villinger,
et al., Real-time monitoring of propofol in expired air in humans undergoing total intravenous anaesthesia, Anesthesiology, 2007, 106, 665–674 CrossRef CAS PubMed
.
- S. Kiem and J. J. Schentag, Interpretation of antibiotic concentration ratios measured in epithelial lining fluid, Antimicrob. Agents Chemother., 2008, 52, 24–36 CrossRef CAS PubMed
.
- Z. J. Zhao, F. Y. He and J. Chen,
et al., Development of Membrane Inlet Based on Ultra Violet Ionization Time of Flight Mass Spectrometer and Its Application in VOCs Analysis at Workplace, J. Chinese Mass Spectrom. Soc., 2021, 42, 16 CAS
.
- F. Maurer, L. Walter and M. Geiger,
et al., Calibration and validation of a MCC/IMS prototype for exhaled propofol online measurement, J. Pharm. Biomed. Anal., 2017, 145, 293–297 CrossRef CAS PubMed
.
- G. Skirbutis, A. Dzingutė and V. Masiliūnaitė,
et al., PEEK polymer's properties and its use in prosthodontics. A review, Stomatologija, 2018, 20, 54–58 Search PubMed
.
- F. Maurer, M. Geiger, T. Volk, D. I. Sessler and S. Kreuer, Validation of liquid and gaseous calibration techniques for quantification of propofol in breath with sorbent tube thermal desorption system GC-MS, J. Pharm. Biomed. Anal., 2017, 143, 116–122 CrossRef CAS PubMed
.
-
D. M. Avishay and K. M. Tenny, Henry's Law, StatPearls Publishing, Treasure Island (FL), 2022 Search PubMed
.
- S. Kamysek, P. Fuchs, H. Schwoebel, J. P. Roesner, S. Kischkel, K. Wolter, C. Loeseken, J. K. Schubert and W. Miekisch, Drug detection in breath: effects of pulmonary blood flow and cardiac output on propofol exhalation, Anal. Bioanal. Chem., 2011, 401, 2093–2102 CrossRef CAS PubMed
.
- W. Miekisch, P. Fuchs, S. Kamysek, C. Neumann and J. K. Schubert, Assessment of propofol concentrations in human breath and blood by means of HS-SPME-GC–MS, Clin. Chim. Acta, 2008, 395, 32–37 CrossRef CAS PubMed
.
- T. Birken, J. Schubert, W. Miekisch and G. Nöldge-Schomburg, A novel visually CO2 controlled alveolar breath sampling technique, Technol. Health Care, 2006, 14, 499–506 Search PubMed
.
- D. Lorenz, F. Maurer and K. Trautner,
et al., Adhesion of volatile propofol to breathing circuit tubing, J. Breath Res., 2017, 11, 036005 CrossRef PubMed
.
- F. Maurer, D. J. Lorenz and G. Pielsticker,
et al., Adherence of volatile propofol to various types of plastic tubing, J. Breath Res., 2017, 11, 016009 CrossRef CAS PubMed
.
- J. W. Sall and J. Leong, Technical communication: stability of propofol in polystyrene-based tissue culture plates, Anesth. Analg., 2013, 117, 65–67 CrossRef CAS PubMed
.
- C. Berchtold, M. Bosilkovska, Y. Daali, B. Walder and R. Zenobi, Real-time monitoring of exhaled drugs by mass spectrometry, Mass Spectrom. Rev., 2014, 33, 394–413 CrossRef CAS PubMed
.
- N. H. G. Holford and L. B. Sheiner, Understanding the dose-effect relationship, Clin. Pharmacokinet., 1981, 6, 429–445 CrossRef CAS PubMed
.
- A. Misra, S. Ganesh, A. Shahiwala and S. P. Shah, Drug delivery to the central nervous system: a review, J. Pharm. Pharm. Sci., 2003, 6, 252–273 CAS
.
- C. Hornuss, D. Wiepcke and S. Praun,
et al., Time course of expiratory propofol after bolus injection as measured by ion molecule reaction mass spectrometry, Anal. Bioanal. Chem., 2012, 403, 555–561 CrossRef CAS PubMed
.
Footnote |
| † Xiaoxiao Li and Pan Chang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |