 Open Access Article
Open Access ArticleComparison of recovery efficiency and matrix effect reduction in pesticide residue analysis: QuEChERS with d-SPE, SPE, and FaPEx in apples and Korean cabbage†
Hyosub
Lee
 *,
Yuran
Cho
,
Geonhee
Jung
,
Hyanghee
Kim
and
Wontae
Jeong
*,
Yuran
Cho
,
Geonhee
Jung
,
Hyanghee
Kim
and
Wontae
Jeong
National Institute of Agricultural Sciences, 166 Nongsaengmyeong-ro, Iseo-myeon, Wanju-gun, Jeollabuk-do 55365, Korea
First published on 11th July 2023
Abstract
QuEChERS is widely used for the analysis of pesticide residues. However, d-SPE, which is commonly utilized in QuEChERS, demonstrates lower clean-up effectiveness than that achieved using conventional SPE, leading to an inadequate reduction of matrix effects. Hence, methods, such as internal standards and matrix-matched calibration (MMC), are frequently employed to address matrix effects. The most effective way to minimize matrix effects is to enhance the clean-up efficiency. In this study, the analytical efficiencies of conventional QuEChERS, d-SPE, SPE, and FaPEx, a novel analytical method, were compared for the clean-up of apple and Korean cabbage samples. The proportion of test pesticides within the appropriate recovery range was 94–99% for QuEChERS, d-SPE, and SPE, while it was 80–95% for FaPEx. When evaluating the recovery results by group, the proportion of pesticides in group III (90–105%) was lower for FaPEx (3–70%) than that for d-SPE (85–92%) and SPE (79–89%). The matrix effect reduction was satisfactory for all clean-up methods, with more than 94% of the test pesticides showing low levels of matrix effects within ±20%. In FaPEx, over 98% of the test pesticides exhibited low matrix effects, indicating better reduction effects than in QuEChERS-based d-SPE and SPE. Method validation results at 0.01 and 0.1 mg kg−1 concentration levels using QuEChERS, SPE (PSA), and FaPEx (amine + C18) demonstrated that more than 95% of the test pesticides were within the appropriate recovery range. Overall, our study contributes to the development of efficient and reliable analytical methods for ensuring the safety and quality of agricultural products.
Introduction
Advanced techniques such as clean-up are needed to accurately analyze trace residues of pesticides in agricultural products. Recently, analytical methods have shifted from single to multiple residue analysis, primarily owing to the application of highly selective analytical methods, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS). Such advanced methods have greatly simplified analytical processes and enabled the detection of even trace amounts of pesticide residues with remarkable accuracy and precision. Quick, easy, cheap, effective, rugged, and safe (QuEChERS) methods have gained considerable popularity. Developed by Anastassiades et al., the QuEChERS approach has two steps: extraction and partitioning using magnesium sulfate (MgSO4), followed by clean-up through dispersive solid-phase extraction (d-SPE).1 QuEChERS offers several notable advantages that have contributed to its widespread adoption in analytical applications. First, it provides higher recovery rates than those obtained using conventional methods.2 Second, its simplified analysis process reduces the overall analysis time, allowing for more efficient experimentation.2 Thirdly, this technique minimizes the use of organic solvents, thereby promoting a more environmentally friendly approach.2 Finally, QuEChERS is user-friendly and can be successfully performed by researchers with varying levels of experience.2However, simplification of the clean-up process allowed matrix effects to emerge as a major source of errors in such advanced analytical techniques. ‘Matrix effects occur when the analyzed pesticide together with other sample components enter the mass spectrometer. This leads to ionization competition, which can either decrease or increase chromatographic signals, adversely impacting the accuracy of quantitative analysis.2–6 Therefore, various attempts have been made to minimize matrix effects. The accuracy of quantitative analysis can be improved when internal standards are used in multi-residue analysis using QuEChERS and LC-MS/MS for agricultural products.7–11 Currently, the method most widely used by analysts to reduce matrix effects is matrix-matched calibration (MMC) in which characteristics of the analytical sample are matched with those of the standard material.12 Previous studies have reported that the utilization of MMC in multi-residue analysis using QuEChERS was effective in correcting the matrix effects.13,14 However, calibration methods using internal standards or MMC cannot be used to correct near LOQ (limit of quantification) levels, because they do not purify impurities that cause matrix effects; instead, they play a corrective role.15,16 Therefore, the most effective approach for mitigating matrix effects is to increase the clean-up efficiency. The d-SPE, a clean-up method, is typically employed along with QuEChERS. Although d-SPE offers multiple advantages, such as faster processing and improved stability, compared to conventional SPE methods, it has a lower clean-up efficiency.17 Among various clean-up techniques, SPE has been recognized for its superior clean-up effects compared to d-SPE, affording higher recovery rates and a more significant reduction in matrix effects.1,13,17–19 Therefore, increasing the clean-up efficiency is likely to be the best option for reducing matrix effects. Various studies have been conducted to develop analytical methods with increased clean-up efficiency.20–24 However, the conventional analytical methods are slow and have low cost-effectiveness than QuEChERS and d-SPE, because the former methods need longer analysis time for improved clean-up. QuEChERS is widely employed in routine pesticide residue analysis, primarily because of its speed and simplicity. Consequently, even if an analytical method with a high clean-up effect is developed, its actual applicability in the field can be enhanced only if it is as simple as QuEChERS and easily accessible to novice analysts.
In this study, the analysis efficiencies of the existing QuEChERS and d-SPE methods, improved QuEChERS with a simplified SPE method, and a recently developed SPE-based one-step fast pesticide extraction (FaPEx) method are compared. FaPEx method was introduced by Taiwan Agricultural Chemicals and Toxic Substances Research Institute.25 The applicability of these methods for multi-component analysis and reduction of matrix effects are also evaluated and compared with those of the current QuEChERS and d-SPE clean-up methods.
Results and discussion
Comparison of recovery efficiency of pesticides in apples using different clean-up methods
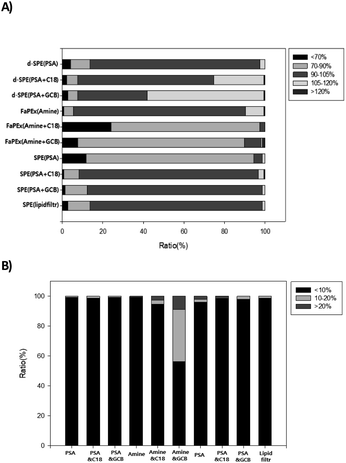
To compare the efficiencies of the recovery rates of different clean-up methods in apples, the test pesticides were added at a concentration of 0.1 mg kg−1. Among 128 tested pesticides, those with an appropriate recovery rate range (70–120%) included 127 for QuEChERS and d-SPE, 123–128 for SPE, and 103–122 for FaPEx. To evaluate the recovery rates of the analytical methods, a group-based evaluation method described elsewhere was used. In our study, recoveries were divided into five distinct groups for ease of evaluation. The acceptable range of 70–120% constituted three groups, each with a width of 15–20%. Additionally, recoveries exceeding the limits of this acceptable range were separated into two further groups. A higher proportion of pesticides in group III (approximately 100% recovery) indicated greater efficiency (Table 1).21,26 The recovery efficiency was in the following order: d-SPE > SPE > FaPEx (Fig. 1).| Group | I | II | III | IV | V |
|---|---|---|---|---|---|
| Criteria (%) | R < 70 | 70 ≤ R < 90 | 90 ≤ R < 105 | 105 ≤ R ≤ 120 | 120 < R |
 | ||
| Fig. 1 Comparison of (A) recovery and (B) RSD effectiveness according to different clean-up methods in apples. | ||
In the three clean-up methods using QuEChERS and d-SPE, the proportion of pesticides in group III was found to be 89–92% (Fig. 1). In previous studies of FaPEx and QuEChERS involving multi-residue analysis (LC-MS/MS) of apples, over 70% of the tested pesticides belonged to group III at this study.26,27 For samples with low contents of impurities, such as sugars, pigments, and lipids, the use of QuEChERS afforded stable analysis results. The proportion of pesticides in group III for QuEChERS and SPE was 5–10% lower than that for d-SPE, while that in groups I and II was 11% higher than that using d-SPE, resulting in a relatively lower recovery rate. SPE-based clean-up methods have lower recovery rates than those achieved using d-SPE, owing to higher interactions between the solvent and pesticides as the solvent passes through the adsorbent.1 However, neither of the analysis methods showed any differences in the acceptable recovery rate (70–120%).
In the FaPEx analysis, the proportion of pesticides in group II (70–90%) was 79–89%, which is higher than those in QuEChERS d-SPE (8–11%) and SPE (5–10%). FaPEx involves the use of an approximately 6 mL syringe filled with absorbents, adsorbents, and filters. It is hypothesized that when 5 mL of the solvent for extracting pesticides from apples is passed through the FaPEx syringe, the volume of the solvent became insufficient to elute all pesticides because of interactions between pesticides and adsorbents. In SPE-based clean-up methods, determining the amount of solvent that can sufficiently elute pesticides is crucial for achieving acceptable recovery rates.28 However, to increase the recovery rates, it is necessary to consider the sensitivity of the analytical equipment, because increasing the volume of the solvent may not meet the LoQ requirements. Chuang et al. (2019) used 1 g of apples and 5 mL of ACN containing 1% acetic acid as the extraction solvent and showed that the FaPEx analysis led to a 50% proportion of pesticides in group II, which was lower than that observed in this study.25 This difference in the results between the two studies is because Chuang et al. (2019) manually controlled the elution rate at one drop per second, whereas this study maintained a 10 psi manifold pressure for elution. SPE clean-up methods, such as FaPEx, are influenced by the experimenter's skills and experimental environment. Thus, the extraction speed of the solvent used in SPE can affect the recovery rate.29 Therefore, accurate analytical guidelines are necessary to enhance the stability and efficiency of FaPEx. Additionally, the adsorbents used in FaPEx differed between the studies; Chuang et al. (2019) mixed primary secondary amine (PSA), C18, and graphite carbon black (GCB), whereas this study used them separately based on their purposes. As a result, different adsorbent compositions can influence the recovery rates.30 Furthermore, in the FaPEx clean-up with added GCB, the proportion of unsuitable recovery rates in groups I and V was 20%, the highest among all clean-up methods. It is well-known that GCB can adsorb compounds with planar structures and strongly adsorb some pesticides, negatively affecting the recovery rates.31 Moreover, its highly porous structure with a large surface area slows down the solvent flow, potentially causing volatile pesticides to evaporate along with highly volatile organic solvents, negatively impacting the recovery rate results.32
In this study, over 99% of the test pesticides (94% for amine + GCB in FaPEx) exhibited relative standard deviation (RSD) values within the appropriate range (<20%). The proportion of pesticides with RSD values below 10% was stable at 98–100% for QuEChERS, d-SPE, and SPE. However, the proportion of amines + GCB in the FaPEx was 58% lower than that for d-SPE and SPE. When the GCB content is high, pesticides may be adsorbed along with impurities, making it difficult to achieve consistent recovery rates for each sample, affecting the RSD.1 For samples with low impurity contents, such as apples, the interactions between GCB and pesticides increased, rendering the use of GCB-containing FaPEx inappropriate.
Comparison of recovery rate analysis results in Korean cabbage
A comparative study of the analytical efficiency according to the clean-up methods for Korean cabbage was conducted using the same methods as used for apples. In Korean cabbage samples, the proportion of pesticides within the appropriate recovery rate range was 94% for QuEChERS and all d-SPE clean-ups, 94–99% for SPE, and 95% for all FaPEx clean-ups (Fig. 2). In contrast to apples, the proportion of pesticides within the inappropriate recovery rate range was the highest when the d-SPE clean-up was performed. The recovery rate improves as the clean-up efficiency increases in samples with a high impurity content.33 The proportion of pesticides within the inappropriate recovery rate range increased because the clean-up effect of d-SPE was lower than that achieved by other methods. | ||
| Fig. 2 Comparison of (A) recovery and (B) RSD effectiveness according to different clean-up methods in Korean cabbages. | ||
The proportion of pesticides in group III was higher for d-SPE (85–88%) than that for the SPE clean-up (83–87%) and FaPEx (9–70%). In particular, d-SPE demonstrated superior performance, producing stable results with a recovery rate close to 100%. The d-SPE method involves shaking the dispersed adsorbent and analytical sample in a centrifuge tube to adsorb impurities and achieve a clean-up. In contrast, SPE involves passing the sample through an adsorbent packed in a syringe, adsorbing impurities, and eluting pesticides. The recovery rate results vary depending on the elution speed and the experimenter's skills. Therefore, SPE clean-up presents difficulties in producing stable results owing to the influence of the experimental environment, solvents used, and elution speed on the recovery rates.
Moreover, d-SPE is known to be more stable than SPE because the impurities in the sample solvent react with the dispersed adsorbent.34 In Korean cabbage samples, the proportion of RSD below 10% was 99–100% for the d-SPE clean-up, whereas SPE clean-up showed a similar result at 98–100%, and that of FaPEx decreased to 70–97%. The proportion of pesticides with an RSD within 20% was confirmed to be stable at over 95% for all clean-up methods.
Comparison of recovery rates between apples and Korean cabbages
In the QuEChERS and d-SPE clean-up, the proportion of pesticides in group III in Korean cabbage samples was 81–84%, which is lower than that in apples (Table S1†). Furthermore, the proportion of pesticides exceeding the recovery rate range in groups I and V was 5–6%, which is lower than the unsuitability rate of less than 1% in the multi-residue analysis in these groups. As the LoQ, extraction efficiency, and clean-up effects may vary depending on the analytical sample, the recovery rates may differ even when using the same analytical method.35 Leaf vegetable like Korean cabbage exhibit higher matrix effects than fruit samples such as apples because of impurities, such as pigments and chlorophyll.31 In a previous study analyzing 240 types of pesticides in apples and lettuce using QuEChERS and LC-MS/MS, the numbers of pesticides at a recovery rate concentration of 45 ng g−1 were 16 and 24, respectively, confirming a lower recovery rate efficiency in leafy vegetables.25 The differences in the impurities of the apple and lettuce samples appeared to affect the recovery rate.In the QuEChERS and SPE clean-up, the proportion of pesticides within the appropriate recovery rate range was 94–99% for Korean cabbage and 96–100% for apples, with the apples exhibiting a higher proportion of pesticides within the appropriate recovery rate range (Table S2†). The proportion of pesticides in group III was similar for both Korean cabbage (83–87%) and apples (79–89%) and was higher in the clean-up with added GCB (86%) for apples (79%). In Korean cabbage, GCB is known to efficiently remove chlorophyll II and minimize the reduction in pesticide-recovery rates, making it suitable for the analysis of leafy vegetables.36 It is believed that chlorophyll II in Korean cabbage adsorbs on GCB in competition with pesticides, resulting in the extraction of more pesticides with the solvent than in apples and a higher recovery rate.
For FaPEx, the proportion of pesticides within the appropriate recovery rate range was 95% for Korean cabbage, which was higher than that observed for apples (91–95%) (Table S3†). Among the used adsorbents in FaPEx, amine and the combination of amine and C18 showed the highest proportions of pesticides in group III (63% and 70%, respectively). In apples subjected to the same clean-up methods, the proportions in group III were 3% and 8%, respectively, showing a lower recovery rate effect than that shown for Korean cabbage. In addition, Korean cabbage was found to have a higher recovery rate, as impurities were sufficiently adsorbed to amines and C18, resulting in less interference between the pesticides and adsorbents compared to apples. For Korean cabbage subjected to GCB clean-up, the proportion of pesticides in group III was 9%, which was not significantly different from that observed in apples (2%). However, the proportion of pesticides within the appropriate recovery rate range was 95%, which is higher than that observed in apples (80%). Moreover, FaPEx, as an SPE-based method, showed a higher recovery rate in Korean cabbage with a high impurity content. The difference in the recovery rates between apples and Korean cabbage was lower in QuEChERS and SPE clean-up than in FaPEx, which was attributed to the difference in the amount of adsorbent. As the amount of the adsorbent increases, the interactions between the analytes in the sample and the adsorbent can affect the recovery rate.37 Based on the results of this study, it can be suggested that obtaining appropriate analysis results may be difficult in samples with low impurity content, such as apples because the number of adsorbents in FaPEx can act as a factor that reduces the recovery rate. However, compared to d-SPE, SPE-based clean-up methods are expected to achieve excellent recovery rates. Thus, superior recovery rates can be obtained for samples with high impurity contents, such as pigments and lipids, by using an appropriate amount of adsorbent.
Comparison of matrix effects
The matrix effects for apples and Korean cabbage showed that over 95% of pesticides had low matrix effects of ±20% or less in all clean-up methods (Fig. 3). The difference between the samples was that the proportion of pesticides in the intermediate level of matrix effects (±20% to ±50%) was 1–3% higher in Korean cabbage than in apples. These results are consistent with previous study findings comparing matrix effects in apples and leafy vegetables, such as spinach, where all test pesticides in apples were within ±20%, while those in spinach ranged from −60 to 20%, indicating higher matrix effects.38 Leafy vegetables are believed to exhibit matrix effects owing to the presence of impurities, such as chlorophyll and color, which are lower in apples with a lower impurity content.39–41 Over 95% of the pesticides tested in both samples exhibited poor matrix effects. Although matrix effects vary depending on the sample, it was inferred that differences in the matrix effect did not occur in this study on apples and Korean cabbage because sufficient clean-up effects were obtained using QuEChERS and d-SPE.18,42,43 | ||
| Fig. 3 Comparison of matrix effects obtained using different clean-up methods in (A) apple and (B) Korean cabbage. | ||
In the FaPEx analysis method, the proportion of pesticides with low matrix effects was 1–3% higher than that in the QuEChERS-based clean-up methods. This is because SPE exhibits a higher clean-up effect than d-SPE, which helps minimize the matrix effects.1,44 Additionally, the amount of sorbent in FaPEx is higher than that in QuEChERS and SPE, resulting in superior clean-up effects with the former. Hence, it can be suggested that using FaPEx to analyze samples with higher impurity contents, such as pigments and lipids, would make the matrix effect lower than that observed with the existing QuEChERS and d-SPE clean-up methods. However, in QuEChERS methods using SPE clean-up, adjusting the sorbent amount rather than adding the same amount as that in d-SPE is necessary. Another approach to enhance the clean-up efficiency is to reduce the diameter of the syringe because it increases contact between the impurities in the sample solvent and the sorbent.
Method validation
To evaluate the applicability of FaPEx, QuEChERS with SPE for routine multi-residue analysis, a comprehensive validation study was performed. The validation criteria took into account recovery rates, RSD, and matrix effects, ultimately identifying amine + C18 and PSA + C18 as the most effective techniques among the tested analytical methods. The pesticides in the samples were spiked at the levels of 0.01 and 0.1 mg kg−1, and the recovery experiments were conducted in triplicate. The LoQ of tested pesticides for Korean cabbage using SPE clean-up was 0.01 mg kg−1, except for pentoxazone. In the QuEChERS and SPE clean-up methods, all pesticides in Korean cabbage, except clethodim, were within the appropriate recovery rate range (Table S4†). However, with FaPEx, probenazole, propamocarb, pyrifluquinazon, and spinetoram exceeded the appropriate recovery rate range for both apples and Korean cabbage. Chuang et al. (2019) reported that multi-residue analysis using FaPEx for mangoes and carrots showed that propamocarb, a pesticide overlapping with this study, was out of the appropriate recovery rate range, whereas spinetoram was included in the appropriate recovery rate.28 Among the pesticides exceeding the appropriate recovery rate range, propamocarb has a solubility of 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 in water, similar to that in the polar organic solvents methanol (933
000 mg L−1 in water, similar to that in the polar organic solvents methanol (933![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and acetone (921
000) and acetone (921![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), which are presumed to have influenced the extraction efficiency and, consequently, the recovery rate. As the number of oxygen atoms in the molecular structure increases, the polarity increases, leading to relatively higher solubility in polar solvents.45 Additionally, when the pH of the sample decreases because of the amine group in the molecular structure, anionic propamocarb can be adsorbed by PSA.
000), which are presumed to have influenced the extraction efficiency and, consequently, the recovery rate. As the number of oxygen atoms in the molecular structure increases, the polarity increases, leading to relatively higher solubility in polar solvents.45 Additionally, when the pH of the sample decreases because of the amine group in the molecular structure, anionic propamocarb can be adsorbed by PSA.
The QuEChERS and SPE methods were considered suitable because 100% and 99% of the tested pesticides in apple and Korean cabbage samples, respectively, were within the appropriate recovery rate range. FaPEx was deemed appropriate for multi-residue analysis with a suitability of over 95%. However, a higher recovery rate can be achieved if the extraction efficiency of FaPEx is improved.
Experimental
Pesticide standard solutions and reagents
The test pesticides were selected based on the monitoring results of the National Agricultural Products Quality Management Service, focusing on those with a high frequency and high residue detection. Pesticide standards used in the analysis were purchased as 1000 mg L−1 stock solutions from Kemidas (Suwon, Korea), and for analytical convenience, the test pesticides were diluted with acetonitrile (ACN) to prepare 10 mg L−1 working solutions. The solvents used in the analysis (i.e., acetonitrile, acetic acid (>99%), and formic acid (>98% purity)) were of HPLC grade (Merck, Darmstadt, Germany). Ammonium formate (99% purity) was purchased from Sigma-Aldrich (St. Louis, MS). Triple-distilled water was obtained using a Milli-Q system (Millipore, Billerica, MA, USA). QuEChERS extraction, partitioning, and d-SPE clean-up reagents were obtained from Agilent Technologies (California, USA). The GCB-added SPE cartridge, LipidFiltr, was purchased from UCP (Bristol, UK). To evaluate the analytical efficiency of the modified clean-up method, magnesium sulfate (MgSO4), PSA, C18, and GCB were purchased from Sigma-Aldrich (St. Louis, MS, USA).Analytical methods for clean-up
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with ACN, transferred to a vial, and analyzed by LC-MS/MS.
1 with ACN, transferred to a vial, and analyzed by LC-MS/MS.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with ACN, and transferred to a vial.
1 with ACN, and transferred to a vial.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with ACN and analyzed using LC-MS/MS according to the instrument analysis conditions.
1 with ACN and analyzed using LC-MS/MS according to the instrument analysis conditions.
Recovery analysis and method validation
Recovery tests were performed to assess the efficiency and reliability of the analytical methods. Recovery tests involved spiking untreated samples at 0.01 and 0.1 mg kg−1 levels. This was achieved by adding 200 μL of the working solution, prepared at concentrations of 0.5 mg kg−1 and 5 mg kg−1, directly to the sample. Since FaPEx is five times more diluted than QuEChERS, pesticide levels were spiked at 0.05 and 0.25 mg kg−1 in the samples, and the recovery tests were performed in triplicate. The limits of detection (LoD) and LoQ were measured based on signal-to-noise (S/N) ratios of 3 and 10, respectively, by analyzing the standard solutions ranging from 0.0005 to 0.01 mg kg−1 using chromatographic instruments.Matrix effects
To compare the degree of matrix effects in apple and Korean cabbage samples according to the pretreatment method, an experiment was conducted using the difference in area values between the standard and matrix-matched solutions. A matrix-matched solution was prepared following the same procedure used for the sample analysis, but without the addition of any pesticide. This ensured the experimental conditions remained consistent throughout the study. Matrix effects were calculated using the following formula (the evaluation standards are listed in Table 2).| Matrix effects (%) | Standard of evaluation |
|---|---|
| ≤|20| | Low effect |
| |20|> and ≤|50| | Medium effect |
| |50|> | High effect |
Conclusions
We conducted a comprehensive comparison of the recovery rates in apple and Korean cabbage samples using 10 distinct clean-up methods: QuEChERS, d-SPE (three types), SPE (four), and FaPEx (three). The efficiency of these analytical methods was assessed by categorizing their recovery rates into five groups (<70%, 70–90%, 90–105%, 105–120%, and >120%). A higher percentage of pesticides within the 90–105% recovery rate (group III) signified superior recovery efficiency. The proportions of pesticides in group III were found to be 85–92% for d-SPE, 79–89% for SPE, and 3–70% for FaPEx. FaPEx displayed the highest proportion of pesticides in the 70–90% recovery range (group II) (21–88%). Given that SPE relies on solvent migration and stronger interactions between the pesticides and adsorbents than d-SPE, a relatively lower recovery rate was observed. Except for the GCB-containing clean-up method, the RSD was within the acceptable range for over 99% of the FaPEx experiments. The implementation of mass spectrometry has made matrix effects critical in the evaluation of analytical efficiency. The most effective strategy for mitigating matrix effects is to enhance the clean-up efficiency. We compared the reduction effects of various clean-up methods. Over 95% of the tested pesticides in both apple and Korean cabbage samples demonstrated low matrix effects across all clean-up methods. FaPEx exhibited 1–3% higher reduction effects than that demonstrated by the QuEChERS, d-SPE, and SPE methods, indicating a greater clean-up efficacy. SPE-based clean-up methods also showed elevated clean-up efficiencies. In addition, substantial differences were found in samples with high levels of impurities. Moreover, we anticipate that the QuEChERS and SPE clean-up methods, when executed in higher proportions, will yield rapid and effective clean-up results akin to those obtained with d-SPE.In conclusion, our study highlights the importance of developing efficient and accurate analytical methods for analyzing agricultural products with diverse matrices. With advancements in analytical techniques and the globalization of agricultural trade, the number of analyzed pesticides is increasing, making it essential to ensure the availability of accurate and rapid analytical methods for the seamless distribution of safe agricultural products. Our findings suggest that not only QuEChERS and d-SPE clean-up but also SPE and FaPEx can be effective methods for analyzing agricultural products with high matrix content. However, further research encompassing a broader range of samples and pesticides is necessary to effectively utilize these methods in routine monitoring and surveillance.
Author contributions
Conceptualization: H.-S. L.; methodology: H.-S. L.; validation: G.-H. J.; formal analysis: Y.-R. C. and H.-H. K.; data curation: H.-S. L. and W.-T. J.; writing – original draft preparation: H.-S. L.; writing – review and editing: H.-S. L. and H-S. L.; supervision: H.-S. L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Research Program for Agriculture Science and Technology Development, Establishment of endosulfan residue standards in ginseng and soil of scheduled sites and development of reduction technology (PJ014487), Rural Development Administration, Republic of Korea. We would like to thank Editage (https://www.editage.co.kr/) for English language editing.Notes and references
- M. Anastassiade, S. J. Lehotay, D. Štajnbaher and F. J. Schenck, Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce, J. AOAC Int., 2003, 86, 412–431 CrossRef.
- B. K. Matuszewski, M. L. Constanzer and C. M. Chavez-Eng, Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS, Anal. Chem., 2003, 75, 3019–3030 CrossRef CAS PubMed.
- T. M. Annesley, Ion suppression in mass spectrometry, Clin. Chem., 2003, 49, 1041–1044 CrossRef CAS PubMed.
- H. Trufelli, P. Palma, G. Famiglini and A. Cappiello, An overview of matrix effects in liquid chromatography-mass spectrometry, Mass Spectrom. Rev., 2011, 30, 491–509 CrossRef CAS PubMed.
- B. K. Matuszewski, M. L. Constanzer and C. M. Chavez-Eng, Strategies for the assessment of matrix effects in quantitative bioanalytical methods based on HPLC-MS/MS, Anal. Chem., 2003, 75, 3019–3030 CrossRef CAS PubMed.
- C. F. Poole and S. K. Poole, Chromatography Today, Elsevier, 2007 Search PubMed.
- Z. Dzuman, M. Zachariasova, O. Lacina, Z. Veprikova, J. Slanina and J. Hajslova, A rugged high-throughput analytical approach for the determination and quantification of multiple mycotoxins in complex feed matrices, Talanta, 2015, 140, 103–110 Search PubMed.
- S. C. Cunha and J. O. Fernandes, Development and validation of a method based on a QuEChERS procedure and heart-cutting gas chromatography–mass spectrometry for determination of polycyclic aromatic hydrocarbons in edible oils, J. Chromatogr. A, 2010, 1217, 2541–2550 Search PubMed.
- M. Asensio-Ramos, J. Hernández-Borges, L. M. Ravelo-Pérez and M. Á. Rodríguez-Delgado, Evaluation of a modified QuEChERS method for the extraction of pesticides from agricultural, ornamental and forestal soils, Anal. Chim. Acta, 2010, 671, 46–57 Search PubMed.
- J. F. Huertas-Pérez, S. Seccia and S. Úbeda, Determination of polar pesticides in olive oil by hydrophilic interaction liquid chromatography and tandem mass spectrometry, Food Chem., 2016, 199, 799–805 CrossRef PubMed.
- A. R. Fernandez-Alba and J. F. Garcia-Reyes, Large-scale multi-residue methods for pesticides and their degradation products in food by advanced LC-MS, TrAC, Trends Anal Chem., 2008, 27, 973–990 CrossRef CAS.
- E. Matisová and M. Dömötörová, Matrix effects in (ultra)trace analysis of pesticide residues in food and biotic matrices, J. Chromatogr. A, 2003, 1000, 181–197 CrossRef PubMed.
- S. J. Lehotay, K. A. Son, H. Kwon, U. Koesukwiwat, W. Fu, K. Mastovska, E. Hoh and N. Leepipatpiboon, Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables, J. Chromatogr. A, 2010, 1217, 2548–2560 CrossRef CAS PubMed.
- S. J. Lehotay and K. Mastovska, Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues, J. Chromatogr. A, 2005, 1067, 207–219 CrossRef PubMed.
- S. M. L. Gómez-Pérez, P. Plaza-Bolaños, R. Romero-González, J. L. Martínez Vidal and A. Garrido Frenich, Comprehensive qualitative and quantitative determination of pesticides and veterinary drugs in honey using liquid chromatography–Orbitrap high-resolution mass spectrometry, J. Chromatogr. A, 2010, 1217, 5872–5885 CrossRef.
- H. Stahnke, S. Kittlaus, G. Kempe and L. Alder, Reduction of matrix effects in liquid chromatography–electrospray ionization–mass spectrometry by dilution of the sample extracts: how much dilution is needed?, Anal. Chim. Acta, 2012, 721, 87–97 Search PubMed.
- T. Rejczak and T. Tuzimski, A review of recent developments and trends in the QuEChERS sample preparation approach, Open Chem., 2017, 15, 100–109 Search PubMed.
- S. J. Lehotay, K. Maštovská and S. J. Yun, Evaluation of two fast and easy methods for pesticide residue analysis in fatty food matrixes, J. AOAC Int., 2005, 88, 630–638 CrossRef CAS PubMed.
- S. J. Lehotay, K. Maštovská and A. R. Lightfield, Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables, J. AOAC Int., 2005, 88, 615–629 CrossRef CAS.
- C. Lesueur, M. Gartner, A. Mentler and M. Fuerhacker, Comparison of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography–mass spectrometry and liquid chromatography–ion trap–mass spectrometry, Talanta, 2008, 75, 284–293 CrossRef CAS PubMed.
- P. Payá, M. Anastassiades, D. Mack, I. Sigalova, B. Tasdelen, J. Oliva and A. Barba, Analysis of pesticide residues using the quick easy cheap effective rugged and safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection, Anal. Bioanal. Chem., 2007, 389, 1697–1714 CrossRef PubMed.
- G. Bystrzejewska-Piotrowska, J. Golimowski and P. L. Urban, Nanoparticles: their potential toxicity, waste and environmental management, Waste Manage., 2009, 29, 2587–2595 CrossRef CAS PubMed.
- S. Niell, V. Cesio, J. Hepperle, D. Doerk, L. Kirsch, D. Kolberg, E. Scherbaum, M. Anastassiades and H. Heinzen, QuEChERS-based method for the multiresidue analysis of pesticides in beeswax by LC-MS/MS and GC×GC-TOF, J. Agric. Food Chem., 2014, 62, 3675–3683 CrossRef CAS PubMed.
- V. Samanidou and S. Nisyriou, Multi-residue methods for confirmatory determination of antibiotics in milk, J. Sep. Sci., 2008, 31, 2068–2090 CrossRef CAS PubMed.
- W. C. Chuang, J. W. Chen, C. H. Huang, T. H. Shyu and S. K. Lin, FaPEx® multipesticide residues extraction kit for minimizing sample preparation time in agricultural produce, J. AOAC Int., 2019, 102, 1864–1876 CrossRef CAS PubMed.
- H. Y. Kwon, C. S. Kim, B. J. Park, Y. D. Jin, K. Son, S. M. Hong and G. J. Im, Multiresidue analysis of 240 pesticides in apple and lettuce by QuEChERS sample preparation and HPLC-MS/MS analysis, Korean J. Pestic. Sci., 2011, 15, 417–433 Search PubMed.
- J. H. Kim, Y. J. Kim, Y. S. Kwon and J. S. Seo, Development of multi-residue analysis of 320 pesticides in apple and rice using LC-MS/MS and GC-MS/MS, Korean J. Pestic. Sci., 2016, 20, 104–127 CrossRef.
- M. EI Badawy, et al., A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis, Anal. Sci., 2022, 38, 1457–1487 CrossRef PubMed.
- C. F. Poole, New trends in solid-phase extraction, TrAC, Trends Anal. Chem., 2003, 22, 362–373 CrossRef CAS.
- L. C. Sander, C. A. Rimmer and W. B. Wilson, Characterization of triacontyl (C-30) liquid chromatographic columns, J. Chromatogr. A, 2020, 1614, 460732 CrossRef CAS PubMed.
- S. J. Lehotay, A. de Kok, M. Hiemstra and P. Van Bodegraven, Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection, J. AOAC Int., 2005, 88, 595–614 CrossRef CAS PubMed.
- C. Lesueur, M. Gartner, A. Mentler and M. Fuerhacker, Comparison of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography–mass spectrometry and liquid chromatography–ion trap–mass spectrometry, Talanta, 2008, 75, 284–293 CrossRef CAS PubMed.
- P. Zhao, L. Wang, L. Zhou, F. Zhang, S. Kang and C. Pan, Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method, J. Chromatogr. A, 2012, 1225, 17–25 CrossRef CAS PubMed.
- T. Tomasz and R. Tomasz, Application of HPLC–DAD after SPE/QuEChERS with ZrO2-based sorbent in d-SPE clean-up step for pesticide analysis in edible oils, Food Chem., 2016, 190, 71–79 CrossRef PubMed.
- M. García-Vara, C. Postigo, P. Palma, M. J. Bleda and M. L. de Alda, QuEChERS-based analytical methods developed for LC-MS/MS multiresidue determination of pesticides in representative crop fatty matrices: olives and sunflower seeds, Food Chem., 2022, 386, 132558 CrossRef PubMed.
- B. Yang, W. Ma, S. Wang, L. Shi, X. Li, Z. Ma and H. Li, Determination of eight neonicotinoid insecticides in Chinese cabbage using a modified QuEChERS method combined with ultra performance liquid chromatography-tandem mass spectrometry, Food Chem., 2022, 387, 132935 CrossRef CAS PubMed.
- M. C. Hennion, Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography, J. Chromatogr. A, 1999, 856, 3–54 CrossRef CAS PubMed.
- H. Kwon, S. J. Lehotay and L. Geis-Asteggiante, Variability of matrix effects in liquid and gas chromatography–mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops, J. Chromatogr. A, 2012, 1270, 235–245 CrossRef CAS PubMed.
- H. Trufelli, P. Palma, G. Famiglini and A. Cappiello, An overview of matrix effects in liquid chromatography-mass spectrometry, Mass Spectrom. Rev., 2011, 30, 491–509 CrossRef CAS PubMed.
- P. J. Taylor, Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry, Clin. Biochem., 2005, 38, 328–334 CrossRef CAS PubMed.
- E. Chambers, D. M. Wagrowski-Diehl, Z. Lu and J. R. Mazzeo, Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses, J. Chromatogr. B, 2007, 852, 22–34 CrossRef CAS.
- M. Misselwitz, S. Lupo, J. Kowalski, R. Lake and J. Cochran, The Promise of Dilute-and-Shoot LC/MS/MS: Feasibility of Dilute-And-Shoot Injections for Pesticide Residue Analysis in Different Food Types Using Experimentally Determined Matrix Effects, Restek Corporation, Bellefonte, 2012 Search PubMed.
- L. Alder, K. Greulich, G. Kempe and B. Vieth, Residue analysis of 500 high-priority pesticides: better by GC-MS or LC-MS/MS?, Mass Spectrom. Rev., 2006, 25, 838–865 CrossRef CAS PubMed.
- Y. Li, Q. An, C. Zhang, C. Pan and Z. Zhang, Comparison of Sin-QuEChERS nano- and d-SPE methods for pesticide multi-residues in lettuce and Chinese chives, Molecules, 2020, 25, 3391 CrossRef CAS PubMed.
- L. R. Snyder, J. J. Kirkland and J. W. Dolan, Introduction to Modern Liquid Chromatography, John Wiley & Sons. Inc., Hobeken, New Jersey, US, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ay00612c |
| This journal is © The Royal Society of Chemistry 2023 |

