Design of a highly sensitive and versatile membrane-based immunosensor using a Cu-free click reaction†
Hiroto
Okuyama
 ,
Yukari
Kodama
,
Kazuya
Takemura
,
Hiroki
Yamashita
,
Yuhei
Oshiba
and
Takeo
Yamaguchi
*
,
Yukari
Kodama
,
Kazuya
Takemura
,
Hiroki
Yamashita
,
Yuhei
Oshiba
and
Takeo
Yamaguchi
*
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8503, Japan. E-mail: yamag@res.titech.ac.jp
First published on 22nd February 2023
Abstract
A highly sensitive immunosensor is developed using membrane pores as the recognition interface. In this sensor, a Cu-free click reaction is used to efficiently immobilize antibodies, and the sensor inhibits the adsorption of nonspecific proteins that degrade sensitivity. Furthermore, the sensor demonstrates rapid interleukin-6 detection in the picogram per milliliter range.
Because the concentration of a biomarker in the human body is generally associated with specific disease processes, the development of quantitative methods for biomolecule detection is in great demand. In particular, point-of-care testing (POCT), which allows disease diagnosis at home or at the bedside without requiring specialized equipment or doctors, is attracting considerable attention as a diagnostic technology, which can save human lives and is economical.1,2 An onsite diagnosis using POCT devices allows clinicians to prescribe appropriate medical treatment and considerably improves patient prognosis. In addition, POCT can be a powerful technique in infectious disease management and may help reduce or prevent further infection within the patient population. Meanwhile, POCT devices exhibit unique performance characteristics: (1) high sensitivity and good signal characteristics to accurately detect various biomarkers, (2) short test time, i.e., rapid capture of biomarkers and signal amplification, and (3) convenience and simplicity of use outside a medical institution.3 The detection system is crucial to satisfy these criteria besides factors such as a superior antigen–antibody pair and amplification mechanism. In general, conventional enzyme-linked immunosorbent assays (ELISAs)4 exhibit relatively high sensitivity, and their access to the recognition sites of biomolecules is a rate-limiting step and a long duration (from hours to days) is required for testing. Immunochromatography5 is another well-known method which is rapid, but its low sensitivity is often problematic. Thus, these underlying sensitivity/rapidity tradeoffs are challenges, and a novel biosensor which can overcome the tradeoffs is needed.
To meet the abovementioned requirements, we recently demonstrated a new concept of an immunosensor for POCT using the small pore space of a porous membrane for molecular recognition (Fig. 1a).6 In this sensor, the capture antibody is immobilized inside the submicron-sized pores with extremely high density, potentially affording high sensitivity. Moreover, through this method, the sensor can rapidly detect the target antigen using analyte flow through membrane pores, and access to the reaction site of biomolecules is highly increased compared with the usual access through diffusion. However, further advanced design of the recognition interface is required for practical use. Our previous study6 used coupling of an active ester in grafted polyacrylic acid (PAAc) and amine in the capture antibody. This is one of the gold standard methods for antibody immobilization; however, it has at least two concerns. First, because the antibody immobilization step is a competitive reaction with the hydrolysis of the active ester group, a concentrated antibody solution is required to achieve high-density immobilization.7,8 To solve this issue, the reaction for receptor immobilization requires high selectivity, high reactivity, and versatility to be deployed in a variety of target/receptor pairs. Second, the residual carboxy group is negatively charged under physiological conditions. Therefore, the sensor surface nonspecifically adsorbs positively charged biomolecules, including antibodies, which directly affects the output signal. Thus, conventional sensors cannot fulfill their potential owing to a low signal-to-noise ratio. Our numerical simulation9 suggests that the developed sensor could reach the pg ml−1 order of sensitivity, whereas conventional sensors are limited to the ng ml−1 order of sensitivity.
Herein, we developed a new membrane-based immunosensor by using a Cu-free click reaction (Fig. 1b). The click reaction is well known as a highly selective and efficient reaction10 and is thus suitable for receptor immobilization. Furthermore, the fabrication of the sensor through click reactions will lead to future development of various receptor-based sensors (e.g., using DNA aptamers, enzymes, and peptides). As a click reaction, Cu(I)-catalyzed Huisgen cycloaddition11 is the most commonly used one; however, its application to the immobilization of entire antibodies is limited because Cu(I) can trigger irreversible aggregation of antibodies.12 Thus, in the present study, a Cu-free click reaction is adopted that does not require Cu(I) as a catalyst and uses a ring-strained alkyne.13 In addition, this immobilization involves the use of poly(glycidyl methacrylate) (PGMA) as the graft polymer, which has advantages such as no interaction with charged biomolecules and reduced adsorption of contaminating proteins on the hydrophilic surface via generated hydroxy groups.14
To demonstrate the detection capability, we developed a sensor targeting interleukin-6 (IL-6). IL-6 is a multipotent cytokine that plays an important role in immune responses, inflammation, bone metabolism, reproduction, arthritis, aging, and neoplasia.15 Monitoring the level of IL-6 protein can help diagnose several diseases such as inflammatory diseases, sepsis,16 and cancer.17 The concentration of IL-6 in blood can increase up to approximately nano molar ranges;18 however, advanced diagnosis of IL-6 in other body fluids that are easier to collect, such as saliva, sweat, or urine, requires considerably higher sensitivity to detect picogram per milliliter ranges.19,20
The sensor was prepared following the protocol shown in Fig. 1c. First, PGMA was grafted via plasma-induced graft polymerization (PIGP; details are shown in the ESI and Fig. S1 and S2†). PIGP enables uniform polymer immobilization under mild conditions.21 The grafted amount affects the performance of the click reaction and membrane permeability. In this study, the grafting ratio ϕ (see ESI†) was controlled at 5%, providing a sufficient number of reactive groups inside the pore without decreasing permeability. Between the steps of PIGP, azidation, and ring-opening reaction, the membranes were evaluated using Fourier transform infrared (FTIR) spectroscopy (Fig. 2a). Notably, a polyethylene (PE) porous-membrane (porosity = 50%, thickness = 25 μm, and average pore diameter = 200 nm) substrate was used for FTIR analysis, while a polyethylene terephthalate (PET; porosity = 17.3%, thickness = 22 μm, and uniform pore with diameter = 1 μm) substrate was used for IL-6 detection because PE exhibits simple FTIR peaks and a quantitative analysis could be performed. After PGMA grafting, a peak associated with the stretching vibration of the carboxy group was observed at 1730 cm−1 and a peak derived from the epoxy group was observed at 900 cm−1. After the reaction with NaN3 and H2SO4, a peak attributed to the azide group was observed at 2100 cm−1, and the intensity of the peak associated with the epoxy group observed at 900 cm−1 decreased, confirming the progress of azidation and the ring-opening reaction of the epoxy group. The conversion of the epoxy group into the azide group was ∼60%, which is sufficient with respect to the amount of antibody to be immobilized. The remaining epoxy groups form diol groups through a ring-opening reaction. This reaction was confirmed by the increment of the stretching peak of hydroxy groups at ∼3200 cm−1. The generation of hydroxy groups helps hydrophilicity at the interface and promotes the formation of a hydration layer, inhibiting nonspecific protein adsorption through interactions, which include hydrophobic interactions and hydrogen bonds. This improvement in hydrophilicity at the interface is confirmed by the measurements of the water contact angle (see ESI and Fig. S3†).
Next, nonselective adsorption properties were evaluated based on the type of graft polymer. The PGMA-based membrane (after the ring-opening reaction) and the PAAc-grafted membrane were immersed in fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA) or FITC-labeled lysozyme in phosphate-buffered saline (PBS), and the amount of adsorbed BSA or lysozyme on the membrane interface was quantified using fluorescence microscopy. Because the pH of PBS was 7.4 in this test, BSA was negatively charged (isoelectric point [pI] = 4.7) and lysozyme was positively charged (pI = 11). Fig. 2b shows the inhibition of the adsorption of both BSA and lysozyme on the PGMA-based membrane whereas the PAAc-grafted membrane considerably adsorbs lysozyme. Lysozyme adsorption on the PAAc-grafted membrane is attributed to an electrochemical interaction between the protein and the carboxy group of PAAc. Thus, for application to different analytes and target molecules in diagnosis, the PGMA-based hydrophilic surface is more effective in inhibiting nonspecific adsorption that results in noise.
Cyclooctyne was introduced into the capture antibody through active ester binding (see Fig. S4 and details of the protocol in the ESI†), and the yield of the click-reactive antibody was 74%. Ab immobilization via the Cu-free click reaction was then performed using the PGMA-based membrane. For the analysis, an FITC-labeled anti-BSA antibody was immobilized as the model antibody, and the fluorescence distribution in the cross-section of the membrane was observed to quantify the bound amount (see Fig. S5 and the experimental protocol in the ESI†). Fig. 2c shows the uniform immobilization of the antibody (green colored) along the thickness direction of the membrane, and each pore is visible via the accumulation of the antibody. The density of the immobilized antibody was quantified as 500 ng cm−2 based on a previous report.6 Notably, this value is similar to that calculated by conventional ELISA (300–600 ng cm−2), however, actual antibody concentration (i.e., the amount of antibody per unit space) was greater than that because the membrane pores possess a high surface area to pore space ratio. In fact, the density of the immobilized antibody in this study was 25 times higher than that determined via ELISA with the same geometric area. Importantly, this method can decrease the required amount of antibody up to 80 μl of 200 μg ml−1 antibody per membrane (diameter = 1 cm), which is only 16% that of the antibody concentration required in the active ester method for the PAAc-grafted membrane (which requires 100 μl of 1 mg ml−1 antibody per membrane). Nevertheless, the amount of immobilized antibody and its distribution to the direction of the membrane cross-section were similar as shown in Fig. 2d. This implies that the antibody was successfully immobilized with high reactivity, and the introduced amount almost reaches a plateau where it is dominated by the steric hindrance of the antibody itself.
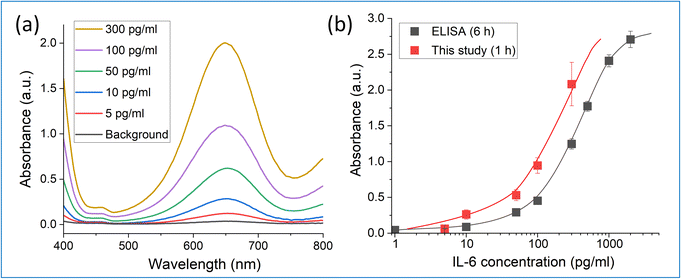
Finally, IL-6 detection tests were performed using the biosensor, in which an anti-IL-6 antibody was immobilized according to the procedure shown in Fig. 1b. Herein, the membrane-based sensor with 1 cm diameter (area = 0.79 cm2) was used in each detection test. The detection protocol comprises the following four steps; permeation of the (1) analyte (antigen solution), (2) biotinylated secondary antibody, (3) enzyme (horseradish peroxidase [HRP])-labeled streptavidin), and (4) substrate (3,3′,5,5′′-tetramethyl benzidene [TMB]) solution using the equipment shown in Fig. S6.† The flow rate was 50 μl min−1 for steps (1)–(3) and 100 μl min−1 for step (4). Based on our numerical simulation,20 almost 100% of the permeated analyte (antigen) is captured in the first step because of the high density of the antibody accumulation inside the pores. However, the reaction rate of the second step was low and a bottleneck for increasing the sensitivity in the membrane-based system. Therefore, in this study, different flow conditions were investigated for the second step and a 30 min flow time, which enables high signal output and low background signal, was adopted. Consequently, the total detection time was ∼60 min (first step = 10 min, second step = 30 min, third step = 10 min, and fourth step = 10 min). In the fourth step, the outlet TMB solution was collected and analyzed using a UV-vis spectrophotometer (HITACHI, UH3900D). Oxidation of TMB was catalyzed by HRP and a blue colored product was formed, whose intensity depends on the amount of bound antigen. Therefore, the absorbance obtained at 650 nm was used as the output signal in this study. Fig. 3a shows the UV-vis signals obtained in the IL-6 detection test. The absorbance observed at 650 nm increased with increasing IL-6 concentration. In addition, the background signal in this test (Fig. 3a, black line) was only 12% compared with that in our previous report.6 This means that the adsorption of the secondary antibody was better inhibited. Antibodies (IgG) can be positively charged in the neutral pH condition,22 and thus, a diol-based polymer layer can effectively inhibit the adsorption of the secondary antibody. Proteins which were examined in this study (BSA, lysozyme, and IgG) may also cause adsorption through hydrophobic interactions or hydrogen bonding. Our graft polymer can suppress these interactions aside from the charge interaction considering that all these adsorptions are suppressed. The performance of the sensor was also compared with conventional ELISA using the same reagents (see ESI†). ELISA required almost 6 h for IL-6 detection when the same antigen and antibody were used (blocking: 1 h, first step: 2 h, second step: 2 h, third step: 20 min, fourth step: 15 min). Thus, the detection time in the membrane-based sensor was only one-sixth of that in ELISA. Fig. 3b shows the comparison of the calibration curves for IL-6 detection using ELISA and the membrane-based biosensor. The membrane-based sensor showed higher signal output than ELISA. Considering that the detection time was considerably reduced compared to that of ELISA, our sensor can detect IL-6 with high sensitivity even in a short period. The limit of detection (LOD) and the limit of quantification calculated from this result were 4.5 and 14.9 pg ml−1 based on 3 SD/slope and 10 SD/slope, respectively, meeting the requirement for the IL-6 biosensor. Furthermore, this sensitivity is considerably superior to that of our previous report (LOD ≈ 1 ng ml−1).6 Difference in the association/dissociation constant in the antigen/antibody system is one of the reasons for this drastic improvement. In addition, usage of the aforementioned diol-based polymer layer and the improved flow condition positively contributed to the decrease in the background signal and enhancement of sensitivity. The performance of the membrane-based biosensor in this study is compared to those of recent IL-6 biosensors in Table 1. We employed a sandwich-type colorimetric detection process, which is usually time-consuming as shown in previous reports.23,24 Nevertheless, this study achieves the LOD required for IL-6 detection in a short test time comparable to those of recent electrochemical label-free sensors.27,28 This shows its potential as a device for POCT, and we intend to expand this sensor into a system using various specimens by optimizing structures of the membrane pore and grafted polymer.
| Method | Sample | LOD (pg ml−1) | Antigen recognition time | Total test time | Ref. |
|---|---|---|---|---|---|
| Colorimetric | PBS | 4.5 | 10 min | 1 h | This study |
| Colorimetric | Serum/buffer | 0.66 | 30 min | ∼2 h | 23 |
| Colorimetric | PBS | 7.8 | 1.5 h | ∼3 h | 24 |
| Fluorescence | PBS | 1 | 2 h | — | 25 |
| Fluorescence | PBS | 7 | 1 h | 2 h | 26 |
| Electrochemical | PBS | 1.6 | 30 min | — | 27 |
| Electrochemical | PBS | 5 | 15 min | 30 min | 28 |
Conclusions
We have developed a membrane-based immunosensor with high sensitivity for detecting IL-6. During sensor fabrication, the capture antibody was efficiently and uniformly immobilized inside small membrane pores through a Cu-free click reaction. In addition, the required antibody was only 16% of the antibody required in the conventional active ester method because of the highly reactive and selective click reaction. Moreover, the grafted polymer with diol groups could inhibit the adsorption of negatively and positively charged biomolecules. This inhibition of adsorption decreased the background signal in the colorimetric detection test. By improving the flow conditions in the test, the sensor showed higher signal output than ELISA and reached an LOD of 4.5 pg ml−1 with a 1 h test duration. Further systematic investigations by using samples such as saliva and serum are now in progress based on the above findings. This study demonstrates the effectiveness of membrane-based immunosensors for detecting low-concentration biomarkers and helps guide designing surface modification methods for the development of a wide range of biosensors.Author contributions
Hiroto Okuyama: writing original draft, conceptualization, validation, and methodology; Yukari Kodama: data curation, formal analysis, and investigation; Kazuya Takemura: data curation and formal analysis; Hiroki Yamashita: data curation and formal analysis; Yuhei Oshiba: methodology and supervision; and Takeo Yamaguchi: project administration, supervision, and resources.Conflicts of interest
There are no conflicts to declare.References
- H. N. Chan, M. J. A. Tan and H. K. Wu, Lab Chip, 2017, 17, 2713–2739 RSC.
- W. H. He, M. L. You, W. T. Wan, F. Xu, F. Li and A. Li, Trends Biotechnol., 2018, 36, 1127–1144 CrossRef CAS PubMed.
- J. J. Liu, Z. X. Geng, Z. Y. Fan, J. Liu and H. D. Chen, Biosens. Bioelectron., 2019, 132, 17–37 CrossRef CAS PubMed.
- R. M. Lequin, Clin. Chem., 2005, 51, 2415–2418 CrossRef CAS PubMed.
- I. Tsuboi and K. Iinuma, Chem. Pharm. Bull., 2021, 69, 984–988 CrossRef CAS PubMed.
- H. Okuyama, Y. Oshiba and T. Yamaguchi, Anal. Chem., 2019, 91, 14178–14182 CrossRef CAS PubMed.
- S. Jeong, J. Y. Park, M. G. Cha, H. Chang, Y. I. Kim, H. M. Kim, B. H. Jun, D. S. Lee, Y. S. Lee, J. M. Jeong, Y. S. Lee and D. H. Jeong, Nanoscale, 2017, 9, 2548–2555 RSC.
- P. Kocbek, N. Obermajer, M. Cegnar, J. Kos and J. Kristl, J. Controlled Release, 2007, 120, 18–26 CrossRef CAS PubMed.
- H. Okuyama, T. Tamaki, Y. Oshiba, H. Ueda and T. Yamaguchi, Anal. Chem., 2021, 93, 7210–7219 CrossRef CAS PubMed.
- W. X. Xi, T. F. Scott, C. J. Kloxin and C. N. Bowman, Adv. Funct. Mater., 2014, 24, 2572–2590 CrossRef CAS.
- J. F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018–1025 CrossRef CAS PubMed.
- M. M. J. Kamphuis, A. P. R. Johnston, G. K. Such, H. H. Dam, R. A. Evans, A. M. Scott, E. C. Nice, J. K. Heath and F. Caruso, J. Am. Chem. Soc., 2010, 132, 15881–15883 CrossRef CAS PubMed.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2005, 127, 11196 CrossRef CAS.
- M. Kim, K. Saito, S. Furusaki, T. Sugo and J. Okamoto, J. Membr. Sci., 1991, 56, 289–302 CrossRef CAS.
- X. P. Jiang, D. C. Yang, R. L. Elliott and J. F. Head, Anticancer Res., 2011, 31, 2899–2906 CAS.
- M. T. Hwang, I. Park, M. Heiranian, A. Taqieddin, S. Y. You, V. Faramarzi, A. A. Pak, A. M. Zande, N. R. Aluru and R. Bashir, Adv. Mater. Technol., 2021, 6, 2100712 CrossRef CAS PubMed.
- J. Joo, D. Kwon, C. Yim and S. Jeon, ACS Nano, 2012, 6, 4375–4381 CrossRef CAS PubMed.
- J. Angulo, C. Martinez-Valdebenito, C. Marco, H. Galeno, E. Villagra, L. Vera, N. Lagos, N. Becerra, J. Mora, A. Bermudez, J. Diaz, M. Ferres and M. Lopez-Lastra, PLoS Neglected Trop. Dis., 2017, 11, e0005757 CrossRef PubMed.
- S. Kumar, J. G. Sharma, S. Maji and B. D. Malhotra, Biosens. Bioelectron., 2016, 78, 497–504 CrossRef CAS PubMed.
- M. D. Hladek, S. L. Szanton, Y. E. Cho, C. Lai, C. Sacko, L. Roberts and J. Gill, J. Immunol. Methods, 2018, 454, 1–5 CrossRef CAS PubMed.
- T. Ito, T. Hioki, T. Yamaguchi, T. Shinbo, S. Nakao and S. Kimura, J. Am. Chem. Soc., 2002, 124, 7840–7846 CrossRef CAS PubMed.
- P. Gupta, E. K. Makowski, S. Kumar, Y. L. Zhang, J. M. Scheer and P. M. Tessier, Mol. Pharm., 2022, 19, 775–787 CrossRef CAS PubMed.
- L. Jiao, H. Y. Yan, W. Q. Xu, Y. Wu, W. L. Gu, H. Li, D. Du, Y. H. Lin and C. Z. Zhu, Anal. Chem., 2019, 91, 8461–8465 CrossRef CAS PubMed.
- L. P. Sun, Y. Huang, T. S. Huang, Z. H. Yuan, W. F. Lin, Z. Sun, M. J. Yang, P. Xiao, J. Ma, W. Wang, Y. Zhang, Z. H. Liu and B. O. Guan, Anal. Chem., 2019, 91, 14141–14148 CrossRef CAS PubMed.
- G. Z. Liu, K. X. Zhang, A. Nadort, M. R. Hutchinson and E. M. Goldys, ACS Sens., 2017, 2, 218–226 CrossRef CAS PubMed.
- X. Hun and Z. J. Zhang, Biosens. Bioelectron., 2007, 22, 2743–2748 CrossRef PubMed.
- C. Oh, B. Park, C. Li, C. Maldarelli, J. L. Schaefer, T. Datta-Chaudhuri and P. W. Bohn, ACS Meas. Sci. Au, 2021, 1, 65–73 CrossRef CAS PubMed.
- R. Cancelliere, A. Di Tinno, A. M. Di Lellis, G. Contini, L. Micheli and E. Signori, Biosens. Bioelectron., 2022, 213, 114467 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed description for sensor fabrication, IL-6 detection test and supporting figures. See DOI: https://doi.org/10.1039/d2ay02110b |
| This journal is © The Royal Society of Chemistry 2023 |