Open Access Article
Open Access ArticleA method for reliable quantification of mercury in occupational and environmental medical urine samples by inductively coupled plasma mass spectrometry
Martin
Winter
 *,
Frederik
Lessmann†
and
Volker
Harth
*,
Frederik
Lessmann†
and
Volker
Harth
Institute for Occupational and Maritime Medicine (ZfAM), University Medical Centre Hamburg-Eppendorf (UKE), Marckmannstraße 129b, Haus 3, 20539, Hamburg, Germany. E-mail: martin.winter@justiz.hamburg.de; Fax: +49(0)40-42731-8778; Tel: +49(0)40-42837-7532
First published on 14th April 2023
Abstract
Over the last years, inductively coupled plasma mass spectrometry (ICP-MS) has been applied as a method for human-biomonitoring of metals in the concentration range of occupational and environmental medicine. In large scale routine monitoring, the determination of mercury (Hg) by ICP-MS remains challenging due to several reasons. Amongst others, stability of dissolved Hg and avoiding memory effects are the key facts for reliable quantification. To address these issues, we developed a robust approach for biomonitoring of mercury in human urine samples by ICP-MS. Using a solution containing HNO3, HCl and thiourea, prepared samples and calibrators were stabilized for up to 72 h. A rinse time of only 30 seconds efficiently prevented contamination of consecutive samples with Hg concentrations up to 30 μg L−1, hence significantly reducing acquisition times compared to published methods. Recovery experiments revealed iridium as an ideal internal standard to compensate matrix effects independently from creatinine concentration. Recoveries of 95.0–104.0% were obtained for Hg levels covering the range of biomonitoring guidance values established by the German Human-Biomonitoring Commission. Excellent intra-day precision and inter-day precision of ≤3.0% for two different Hg levels were achieved. The detection and quantification limit accounted for 21.7 ng L−1 and 65.6 ng L−1, respectively, enabling reliable quantification even in the range of environmental background exposures. Additionally, the method was externally validated by successful participation in the inter-laboratory comparison program G-EQUAS. With the developed method, we hence provide a sensitive and robust tool for mercury exposure assessments in future large scale human-biomonitoring studies.
Introduction
Mercury (Hg) is an element with high toxicity occurring in the environment and a variety of occupational settings.1,2 Due to its ubiquity, humans are more or less constantly exposed to mercury, whereby the extent of adverse health effects depends on the chemical form as well as the dose and time span of exposure.3,4 In environmental and occupational medicine, individual exposure levels can be determined via human-biomonitoring (HBM) by directly measuring Hg in biological samples such as urine and blood. Whereas the latter contains predominantly the organic form, urine contains principally only inorganic mercury.5 Comparison of Hg amounts to health-related guidance values like the HBM values established by the German Human-Biomonitoring Commission or biomonitoring equivalents allows robust risk assessment and sound medical treatment if necessary.6Over the last 20 years the application of inductively coupled plasma mass spectrometry (ICP-MS) for element analysis in large scale biomonitoring has increased,7–11 due to its outstanding performance concerning linearity and sensitivity as well as the simultaneous detection of several elements in a single acquisition. In addition, sample preparation can often be carried out by a simple dilution step.
ICP-MS can be applied easily for many elements, but the analysis of mercury remains challenging for several reasons. The comparatively high first ionization energy (IE = 10.44 eV) causes that only about 4% of the injected Hg amount is ionized,12 leading to reduced sensitivity in mass spectrometric detection. Since mercury occurs naturally in seven isotopes, all with relative abundances less than 30%, sensitivity is even more compromised. Hg analysis is further affected by the element's high volatility and affinity to adsorb to the surface of vessels and sample introduction components, e.g. tubes or the nebulizer chamber, resulting in low recovery rates and sample contamination due to memory effects.
Whereas sensitivity issues can be compensated by modern instruments, stabilizing mercury in urine samples is the major goal to obtain reliable quantitative results. To address this issue, several different sample preparation protocols have been developed. Usually, samples are diluted 5–20 fold with nitric and/or hydrochloric acid.13–20 In some protocols, certain amounts of modifiers like potassium dichromate, gold chloride, ethylenediaminetetraacetic acid (EDTA), cysteine and thiourea are added in various combinations to prevent volatilization and adsorption.21–25 Nevertheless, extensive rinsing of the introduction system after each sample remains necessary to avoid memory effects,15,22,24 leading to prolonged acquisition times.
Apart from element specific challenges, ICP-MS analysis is generally susceptible to spectral and non-spectral interferences. The latter is caused by differences in viscosity and contents of total dissolved solid (TDS) between the sample matrix and calibration standards, effecting the transportation and ionization conditions in the plasma. In laboratory routine, the use of internal standardization is often preferred over standard addition methods to compensate for these effects, due to its relatively simple and fast application. Though, the correct choice of an internal standard (IS) might be difficult, since the isotopic mass and/or ionization energy should be similar to the analyte's.26–28 Furthermore, the IS must be of high purity and free from spectral interferences.
In ICP-MS analysis spectral interferences are predominantly caused by isobaric polyatomic species generated in the plasma by numerous reactions between analytes, matrix components, reagents used for sample preparation and the plasma gas.29 These interferences can be effectively minimized by application of collision cell technology (CCT) and kinetic energy discrimination (KED) before introduction to the mass spectrometer. The collision cell is filled with an inert gas (e.g. Helium), which collides with ions traversing the cell. Due to a larger collision cross section polyatomic species experience more collision events than the corresponding analyte ions resulting in different kinetic energies. A potential barrier between collision cell and mass analyzer hampers ions moving more slowly from entering the quadrupole (KED), hence reducing polyatomic interferences.30
Albeit these challenges ICP-MS constitutes a promising tool for Hg determination in urine samples as demonstrated in previous studies. Developing a sample preparation method capable of stabilizing dissolved Hg amounts efficiently will be critical for reliable quantification. According to the literature, using complexing agents seems to be suitable to avoid vaporization and adsorption. Furthermore, Hg determination can be expected to be compromised by spectral and non-spectral interferences due to the variability and complexity of the urine matrix. Combining modern technologies, e.g. CCT, and the usage of internal standardization it might be possible to compensate these matrix effects.
In this study, we developed a robust sample preparation method for quantitative Hg analysis by ICP-MS in both occupational and environmental medical urine samples. Since the derivation of reference values for biomonitoring requires investigating larger cohorts, long-term stability of mercury in prepared calibration standards and samples was a core aspect in method development. In addition, the influence of different IS on the accuracy of Hg determination depending on the urinary concentration was investigated.
Materials and methods
Chemicals
Nitric acid 67–69%, hydrochloric acid 32–35% (both ultrapure, NORMATOM®) and thiourea (≥99%, analytical reagent) were purchased from VWR (Germany). Triton X-100 (molecular biology grade) was obtained from Sigma Aldrich (Germany). Standard solutions of bismuth (10 mg L−1; 2% HNO3), iridium (100 mg L−1; 2% HCl), were bought from Fluka (Germany). Standard solutions of terbium, yttrium, scandium (each 100 mg L−1; 2% HNO3) were purchased from VWR (Germany) and a standard solution of mercury (10 g L−1; 5% HNO3) from Alfa Aesar (Germany). All elemental solutions were of ICP-MS grade. De-ionized water with >18 MΩ cm resistivity was provided by an in-house Milli-Q® Reference system (Millipore, USA) and was used for the preparation of all solutions, calibrators and samples. Helium gas and Argon (both 99.999%) were purchased from Westfalen AG (Germany).Samples
Commercially available ClinChek® urine control material for trace elements was purchased from Recipe (Germany). Urine samples in the occupational and environmental concentration range were obtained from the inter-laboratory comparison program German External Quality Assurance Scheme (G-EQUAS) managed by the Institute for Occupational, Environmental and Social Medicine (IPASUM) at the University Erlangen-Nuremberg, Germany. For recovery experiments, pooled urine samples were spiked with different amounts of mercury in the range of the German reference and HBM values.Sample preparation, calibration solutions and internal standard
In the final method, 500 μL urine were diluted 5 fold (final volume 2.5 mL) with diluent A (5% HNO3, 0.625% HCl, 0.25% thiourea, w v−1) and incubated for 2 h at room temperature while constantly shaking using an overhead shaker. Incubation was conducted in 15 mL metal free polypropylene tubes sealed with polyethylene screw caps (Carl Roth, Germany). The Hg concentrations of calibration solutions were 0, 0.02, 0.05, 0.1, 0.5, 1, 5, 10 and 30 μg L−1 to obtain linear calibration curves with an coefficient of determination (R2) ≥0.9999 (Fig. 1). Calibrators were prepared by serial dilution of a 10 g L−1 standard solution with diluent A. To minimize physico-chemical differences between calibrators and prepared samples, it was necessary to account for the final concentration of acids and modifier in the prepared solutions (4% HNO3, 0.5% HCl and 0.2% thiourea, w v−1). Hence, we added respective amounts of the pre-diluted standard solution to 2 mL ultra-pure water and filled up with diluent A to a final volume of 10 mL. An internal standard solution was prepared with a final concentration of 800 μg L−1 for scandium (Sc), yttrium (Y), terbium (Tb), iridium (Ir) and bismuth (Bi) in 4% HNO3 (w v−1) with 0.1% Triton-X 100 (v v−1). The solution was added online to all samples, blanks and calibration standards. This method was used throughout the study unless stated otherwise. For the investigation of Hg stabilization, we additionally applied two other diluents B (5% HNO3, 0.625% HCl, w v−1) and C (5% HNO3, w v−1). | ||
| Fig. 1 Calibration curve used for mercury analysis. Calibration solutions were prepared with diluent A. Acquisition was conducted in He mode with internal standardization. | ||
Instrumentation
All experiments were performed using an Agilent 7800 ICP-MS system equipped with SPS 4 autosampler (Agilent Technologies, Japan). Standard nickel sampler and skimmer cones were used. The aerosol was formed in a Scott type spray chamber (double pass, quartz) using a Micro Mist® nebulizer (U-series, glass). Acquisition of the isotopes 201Hg, 45Sc, 89Y, 159Tb, 193Ir and 209Bi was carried out by applying CCT (He mode, 5 mL min−1). Instrument tuning and performance check was conducted according to the manufacturer's guidelines before each measurement. Detailed information on the ICP-MS operating conditions are given in Table 1. All data were recorded and processed with the Agilent MassHunter Workstation software (version 4.5).| Operating Conditions | Values |
|---|---|
| Plasma | |
| RF power | 1550 W |
| RF matching | 1.30 V |
| Sample depth | 8 mm |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Sample introduction | |
| Nebulizer gas | 1.07 L min−1 |
| Nebulizer pump | 0.1 rps |
| Spray chamber cooling temperature | 2 °C |
| Cell gas mode | He |
| He flowrate | 5 mL min−1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Pump program | |
| Sample uptake | 27 s/0.5 rps |
| Stabilization | 45 s/0.1 rps |
| Probe rinse | 10 s/0.5 rps |
| Rinse | 30 s/0.5 rps |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Tubing | |
| Sample | 1.02 mm i.d |
| Internal standard | 0.25 mm i.d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Spectral mode | |
| Peak pattern | 1 point |
| Replicates | 3 |
| Sweeps/Replicate | 100 |
Stabilization of Hg
Lyophilized ClinChek® (level I & level II) urine samples were dissolved in 10 mL de-ionized water according to the manufacturer's instructions. Each sample material was 5 fold diluted with diluent A, B and C (final volume 5 mL). Incubation was conducted for 2 h, 4 h and 24 h at room temperature as described before. After the first acquisition, samples and calibrators prepared with diluent A were stored unsealed at room temperature in the autosampler unit for different periods: urine samples = 24 h, 48 h and calibrators = 24 h, 48 h, 72 h. Reacquisition for each storage period was carried out against freshly prepared calibration solutions.Precision, limit of detection (LOD) and limit of quantification (LOQ)
Lyophilized ClinChek® (level I & level II) urine samples were reconstituted as described before (see Stabilization of Hg). For intra-day precision (n = 10) 500 μL aliquots were freshly prepared and for inter-day precision (n = 10) further aliquots were stored at −20 °C in 15 mL metal free tubes until the day of measurement (maximum storage = 31 days). Thawing was conducted for approximately 15 min at ambient temperature before preparation with diluent A. The LOD and LOQ were calculated considering standard deviation of 10 blank samples, measured directly after the highest calibrator (30 μg L−1) on different days, divided by the slope of calibration curve and multiplied by 3.3 and 10, respectively, and then multiplied by the dilution factor.31Accuracy and evaluation of internal standardization
For recovery experiments human pool urine samples with different creatinine levels were selected: 0.15, 0.42, 0.74, 0.94, 1.35, 1.75, 1.99, 2.45 and 3.22 g L−1. Hg solutions containing 10 μg L−1 (low), 100 μg L−1 (medium) and 500 μg L−1 (high) were prepared by diluting a 10 g L−1 standard solution with diluent A. For each urine sample 500 μL aliquots were spiked with 50 μL of solution low, medium and high and filled up with diluent A to a final volume of 2.5 mL (5 fold dilution). The resulting Hg concentrations for undiluted samples were 1, 10 and 50 μg L−1, respectively. In order to correct recoveries for native Hg backgrounds, each urine sample was also prepared and analyzed without spiking any Hg (same final volume of 2.5 mL). For evaluation of internal standardization the recoveries obtained by using 45Sc, 89Y, 159Tb, 193Ir and 209Bi as IS were compared.Results and discussion
Selection of Hg isotope
Urine constitutes a complex matrix with variable composition, hence increasing the risk of individual polyatomic interferences to occur. In order to minimize potential interferences we generally applied the He mode in contrast to other methods using No-Gas mode.3,32 The sensitivity of Hg analysis is compromised by the element's high ionization energy and the low abundance of its naturally occurring isotopes. Therefore, it seems reasonable to measure the most abundant isotopes 200Hg (23.10% abundance) or 202Hg (29.86% abundance).16,21,24 Unfortunately, in occupational medicine tungsten (W) can be a toxicologically relevant parameter excreted via urine which causes polyatomic interferences for both isotopes (184W16O and 186W16O) even in He mode.12 Lacking such interferences, we decided to use the isotope 201Hg (13.18% abundance) for all measurements. In order to compensate for the loss of sensitivity due to the isotope's lower abundance, the distance between torch and sample cone was reduced. Changing the sampling depth from 10 mm to 8 mm increased the counts per second rate by a factor of about 1.5 (data not shown).Stabilization of Hg
Determination of Hg in urine samples by ICP-MS is challenging due to the element's high volatility and adsorption affinity. Thus, stabilization of dissolved Hg is crucial in sample preparation to avoid poor recovery and memory effects.In a first set of experiments, we investigated the efficiency of Hg stabilization in urine control samples (ClinChek® level I & level II) for diluent A, B and C. Therefore, ICP-MS analysis was conducted after 2 h, 4 h and 24 h of incubation. The obtained relative recoveries based on the manufacturer's specified mean target value were 111.1–113.3% for level I and 110.0–110.6% for level II when using diluent A (Fig. 2). Diluent B resulted in recoveries of 27.5–41.0% for level I and 10.2–21.2% for level II. When sample preparation was carried out using diluent C recoveries of 37.5–56.6% and 19.7–23.6% were obtained for level I and level II, respectively. The results revealed that only diluent A is capable of stabilizing Hg effectively.
Kalamegham et al.24 stated in their study that the sole use of nitric acid as diluent in mercury quantification proved useless because of poor and erratic recoveries even for higher acid concentrations. Since HNO3 is still used in more recent studies,18,19 we investigated its application using diluent C and confirmed the observations made by Kalamegham's workgroup. Interestingly, the addition of hydrochloric acid (diluent B), which is commonly used to stabilize Hg by forming the soluble [HgCl4]2− complex,33 had no impact on recovery. Testing HCl concentrations up to 1% (w v−1) resulted in poor recovery, too (data not shown). A possible explanation might be that the investigated samples contain further elements comparable to real study samples with co-occurring exposures. Elements such as silver (Ag), gold (Au), platinum (Pt) and palladium (Pd) might compete with Hg for chloride ions, thereby hindering formation of the [HgCl4]2−-complex. Using HCl concentrations >1% (w v−1) might improve recoveries, but would dramatically shorten the lifetime of the nickel cones and was hence not investigated any further. Anyway, adding thiourea (diluent A) instead enhanced the recovery significantly. This effect can be explained most likely by mercury's strong thiophilic nature. The underlying high affinity for sulfur might result in formation of a stable and soluble mercury-thiourea-complex similarly to an ion chromatography method for Hg determination in environmental matrices developed by Shade and Hudson.34 Other sulfuric compounds may be suitable as well, but preliminary tests using L-cysteine for example, resulted in unsatisfactory recoveries (data not shown). Hence, we identified thiourea as the ideal modifier for Hg determination in human urine. Furthermore, the obtained data demonstrate that Hg was stabilized for at least 24 h, since recovery did not decrease with increasing incubation times (Fig. 2). This indicates the diluent's potential for the application in large scale studies, where long-term stability of the target analyte is crucial.
During an acquisition, samples and calibrators are usually stored unsealed in the autosampler unit once preparation is completed. Whereas the first samples are acquired after a few minutes, the last samples may be analyzed several hours later, depending on the cohort's dimension. Thus, Hg volatilization and/or adsorption during the storage may affect analysis. Another important aspect is, that the instrument's performance decreases with increasing runtime due to matrix deposits on the cones and lenses. This effect can be monitored by measuring quality control (QC) samples after every few samples. In case of exceeding certain QC tolerance levels defined by the user, the instrument needs to be recalibrated. Recalibration is usually conducted by reacquisition of the solutions initially used for calibration, especially in fully automated acquisition setups. In order to obtain reliable results for all samples, it is hence essential to ensure Hg stabilization over time.
Accordingly, long-term stability of Hg was investigated for both the calibration solutions and urine samples using diluent A. Analyzed samples and calibrators of the previous experiment were reacquired after different storage periods against freshly prepared calibration solutions, hence simulating conditions of large cohort measurements. Recoveries of 111.0–117.1% were obtained for ClinChek® level I samples, 110.0–112.8% for level II and 90.0–105.4% for the calibrators without observing any trends for the different storage periods (Table 2). Our results demonstrate, that diluent A is capable of preserving urine samples for at least 48 h and calibration solutions for 72 h, hence paving the way for large scale measurements without alteration of mercury levels over time. Interestingly, stabilization of Hg was achieved without the use of carcinogenic dichromate22 or gold.21,25 Since the latter is known to possess adsorption affinities similar to Hg,35 the developed method may allow the simultaneous quantification of both elements. This might help to shed further light into the health risks of exposures associated with electronic waste,36 but needs to be investigated in future studies.
| (a) | Incubation time | Recovery [%] for storage time | ||
|---|---|---|---|---|
| Control sample | Initial | 24 h | 48 h | |
| Level I | 2 h | 113.3 | 112.7 | 111.1 |
| 4 h | 114.1 | 111.7 | 114.2 | |
| 24 h | 114.0 | 111.0 | 117.1 | |
| Level II | 2 h | 110.6 | 110.8 | 110.0 |
| 4 h | 111.4 | 110.3 | 110.8 | |
| 24 h | 111.0 | 111.5 | 112.8 | |
| (b) | Recovery [%] for storage time | |||
|---|---|---|---|---|
| Calibrator [μg L−1] | Initial | 24 h | 48 h | 72 h |
| 0.02 | 105.0 | 90.0 | 105.4 | 104.3 |
| 0.05 | 100.0 | 104.0 | 97.3 | 99.0 |
| 0.10 | 102.0 | 98.0 | 93.3 | 101.0 |
| 0.50 | 99.0 | 98.8 | 99.8 | 100.2 |
| 1.00 | 99.2 | 98.1 | 96.9 | 99.2 |
| 5.00 | 100.2 | 98.1 | 98.7 | 98.6 |
| 10.0 | 99.8 | 99.6 | 98.8 | 98.8 |
| 30.0 | 100.0 | 98.6 | 99.8 | 99.4 |
Memory effect
The memory effect is a well-known issue affecting Hg determination by ICP-MS. In order to avoid contamination of consecutive samples, the introduction system needs to be rinsed thoroughly after each sample. Published rinse times range from 85 s to 180 s and seem to depend on the applied rinse solution (Table 3). In our study we investigated the memory effect using diluent A as rinse solution. Therefore, blanks (n = 10, each on a different day) were measured directly after the 30 μg L−1 calibrator which conforms a relatively high Hg concentration expected in 5 fold diluted human urine samples. Rinsing the introduction system for 30 s resulted in estimated Hg concentrations ≤12.2 ng L−1 for the blank material. Since the measured amounts were below the LOD (see following section), the acquisition is not affected by any memory effect. In addition, the rinse time was reduced at least by a factor of 3 compared to published rinse times (Table 3), hence improving sample throughput. Generally, increasing the acquisition speed lowers the analytical costs due to reduced solvent and gas consumption.Precision, LOD and LOQ
The precision of the presented analytical method was evaluated by measuring ClinChek® urine control samples (level I & level II). Intra-day precision was determined by measuring freshly prepared aliquots of both levels and resulted in 0.8% and 0.9% for level I and level II, respectively (Table 4). Inter-day precision was determined over a time span of 31 days and resulted in 2.6% for level I and 3.0% for level II. Interestingly, in contrast to the manufacturer's recommendation to only use freshly prepared control samples, we were able to use frozen aliquots from a single ClinChek® solution without any loss of mercury, hence lowering the material costs. The LOD and LOQ were calculated to be 21.7 ng L−1 and 65.6 ng L−1, respectively. Thus the presented method will not only allow Hg quantification of occupational medical samples, but also in the range of German median environmental medical background levels (approximately 0.2 μg L−1).37| Urine control sample | ||
|---|---|---|
| Level I | Level II | |
| Intra-day (n = 10) | ||
| Mean conc. [μg L−1] | 2.31 | 15.8 |
| RSD [%] | 0.8 | 0.9 |
| Inter-day (n = 10) | ||
| Mean conc. [μg L−1] | 2.30 | 15.8 |
| RSD [%] | 2.6 | 3.0 |
Accuracy and evaluation of internal standardization
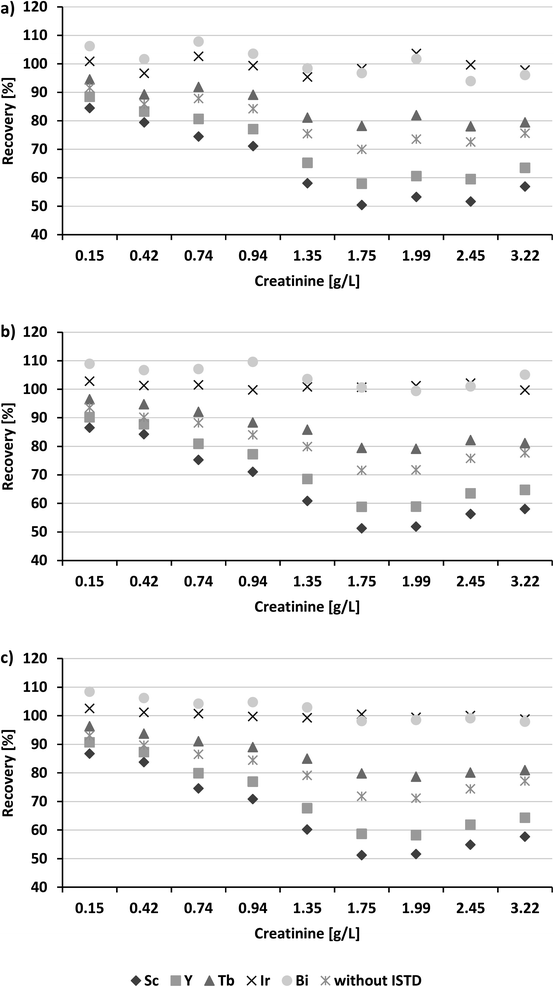
ICP-MS analysis is commonly affected by the sample matrix, due to different physico-chemical properties compared to solvent-based calibrators. Urine constitutes a complex sample matrix containing several different compounds, e.g. proteins, metabolites, urea, bicarbonate, sodium, potassium and chloride. In addition, the composition and concentration can vary, amongst others depending on the patient's diet. Thus, each sample consists of an individual matrix impacting quantification. Albeit frequently discussed limitations,38,39 urinary creatinine is a biomarker used as an estimate to account for concentration of urine samples in human-biomonitoring. According to guidelines of the World Health Organization (WHO) 0.3–3.0 g L−1 creatinine is recommended to perform toxicological evaluations in the field of occupational medicine.40 The German Human-Biomonitoring Commission recommends an even narrower range of 0.5–2.5 g L−1 for environmental biomonitoring studies.41To account for varying urinary concentrations (going along with differing matrix effects), pooled urine samples with 0.15–3.22 g L−1 creatinine were selected for recovery experiments. Samples were spiked with three levels of Hg (low = 1 μg L−1, medium = 10 μg L−1, high = 50 μg L−1), covering the range of relevant guidance values (German reference value = 1 μg L−1, HBM-I = 7 μg L−1, HBM-II = 25 μg L−1).42–44 Independently of the creatinine content, recoveries of 95.0–104.0% were obtained for all spiking levels (Table 5).
| Creatinine [g L−1] | Native Hg [μg L−1] | Measured Hg [μg L−1] | Recovery [%] | ||||
|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | ||
| 0.15 | 0.01 | 1.02 | 10.29 | 51.26 | 101.0 | 102.8 | 102.5 |
| 0.42 | 0.02 | 0.98 | 10.15 | 50.63 | 96.0 | 101.3 | 101.2 |
| 0.74 | 0.33 | 1.35 | 10.48 | 50.70 | 102.0 | 101.5 | 100.7 |
| 0.94 | 0.09 | 1.08 | 10.07 | 49.96 | 99.0 | 99.8 | 99.7 |
| 1.35 | 0.06 | 1.01 | 10.14 | 49.68 | 95.0 | 100.8 | 99.2 |
| 1.75 | 0.17 | 1.15 | 10.24 | 50.42 | 98.0 | 100.7 | 100.5 |
| 1.99 | 0.09 | 1.13 | 10.22 | 49.80 | 104.0 | 101.3 | 99.4 |
| 2.45 | 1.87 | 2.87 | 12.09 | 51.89 | 100.0 | 102.2 | 100.0 |
| 3.22 | 0.15 | 1.13 | 10.12 | 49.54 | 98.0 | 99.7 | 98.8 |
Since recoveries did not correlate with the urinary creatinine content, the results indicate that non-spectral interferences were effectively compensated by using 193Ir as internal standard. This was further supported when recoveries were compared to the corresponding values obtained without internal standardization (Fig. 3), where recovery decreased with increasing amounts of creatinine for all three spiking levels. The criteria for selecting the ideal IS for ICP-MS analysis are being discussed divergently: some authors recommended choosing the IS based on similar isotopic masses,28 whereas others reported that both similarities of the isotopic mass and the first ionization energy are crucial.26,27 Therefore, we additionally evaluated the efficiency of 45Sc (IE = 6.54 eV), 89Y (IE = 6.38 eV), 159Tb (IE = 5.85 eV) and 209Bi (IE = 7.29 eV) to correct for matrix effects. The graphics in Fig. 3 illustrate that for the four elements, Hg recovery correlated with the urinary creatinine content similarly to the data without IS correction. In general, recoveries improved with increasing isotopic mass of the IS. For 209Bi, recoveries even close to 193Ir (IE = 9.10 eV) were obtained, but were not consistent for the investigated creatinine range. This can be explained by the elements' differing ionization energies, whereby the IE of 193Ir is closer to the IE of 201Hg (IE = 10.44 eV), hence supporting the theory that isotopic masses as well as ionization energy similar to the analyte are essential for selecting the ideal IS. Another aspect contradicting the application of 209Bi as an internal standard in human-biomonitoring is, that urine samples might be contaminated with bismuth due to medication with bismuth subsalicylate prior to sampling (e.g. used in “Pepto-Bismol®”, an over-the counter-drug commonly used in the United States and other countries to treat nausea, heartburn and diarrhea). The results demonstrate that only internal standardization with 193Ir allows reliable and robust quantification of Hg in the range of relevant guidance values regardless of the urinary concentration (Fig. 3).
External quality assurance
For external quality control, the developed method was used to participate in the German External Quality Assurance Schemes (G-EQUAS) organized by the University of Erlangen-Nuremberg (EQUAS round no. 66, no. 67 and no. 68). The urine samples in G-EQUAS cover Hg amounts in the occupational and environmental medical concentration range. Applying the ICP-MS method developed in this study resulted in recoveries of 78.6–105.0%, hence fulfilling the required accuracies (Table 6). This demonstrates the method's performance as well as the potential to obtain reliable results even below the current German reference value (1 μg L−1).| EQUAS material | Level | Round | Target [μg L−1] | Measured [μg L−1] | Recovery [%] | Tolerance [μg L−1] |
|---|---|---|---|---|---|---|
| Occupational | Low | No 66 | 6.6 | 6.4 | 97.0 | 4.5–8.7 |
| No 67 | 4.0 | 3.2 | 80.0 | 2.8–5.2 | ||
| No 68 | 4.8 | 4.7 | 97.9 | 3.3–6.3 | ||
| High | No 66 | 14.2 | 13.3 | 93.7 | 10.6–17.8 | |
| No 67 | 29.9 | 31.4 | 105.0 | 23.3–36.5 | ||
| No 68 | 17.1 | 16.3 | 95.3 | 12.9–21.3 | ||
| Environmental | Low | No 66 | 0.46 | 0.39 | 84.8 | 0.22–0.70 |
| No 67 | 0.28 | 0.22 | 78.6 | 0.13–0.43 | ||
| No 68 | 0.10 | 0.09 | 90.0 | 0.04–0.16 | ||
| High | No 66 | 1.16 | 1.11 | 95.7 | 0.77–1.55 | |
| No 67 | 0.74 | 0.73 | 98.6 | 0.50–0.98 | ||
| No 68 | 0.39 | 0.39 | 100.0 | 0.24–0.54 |
Conclusion
In this study we developed a sensitive and robust method for the quantification of Hg in urine samples by ICP-MS, using a specific mixture of nitric acid, hydrochloric acid and thiourea as diluent and rinse solution. Iridium was identified as an ideal IS to compensate for matrix effects independent from urinary concentration. Samples and calibrators are stable over several hours allowing large scale measurements without any loss of mercury, hence paving the way for fully automated acquisitions. Albeit avoiding carcinogenic modifiers such as dichromate, the rinse time could be reduced without observing any memory effect, thereby improving safety, shortening acquisition time and saving resources. In addition, quantification of other highly adsorptive elements, e.g. gold, might be realized by applying the presented sample preparation. However, further studies are required to verify whether this approach can be extended to simultaneous determination of other toxicologically relevant elements in urine samples. Overall, our results demonstrate the method's ability to obtain reliable results in the occupational as well as the environmental medical concentration range. The developed method can hence be applied in future large scale human-biomonitoring studies as a robust tool for individual Hg exposure assessments.Author contributions
Martin Winter: conceptualization (equal); methodology (lead); investigation (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review editing (equal). Frederik Lessmann: conceptualization (equal); methodology (supporting); supervision; validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review editing (equal). Volker Harth: writing – original draft (supporting); writing – review editing (equal).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Jolanta Sikora for conducting the determination of creatinine amounts in pooled urine samples used for recovery experiments.References
- F. Beckers and J. Rinklebe, Crit. Rev. Environ. Sci. Technol., 2017, 47, 693–794 CrossRef CAS.
- J. Gerding, C. Peters, W. Wegscheider, J. Stranzinger, F. Lessmann, K. Pitzke, V. Harth, U. Eickmann and A. Nienhaus, Int. Arch. Occup. Environ. Health, 2021, 935–944 CrossRef CAS PubMed.
- C. C. Bridges and R. K. Zalups, J. Toxicol. Environ. Health, Part B, 2010, 13, 385–410 CAS.
- B. J. Ye, B. G. Kim, M. J. Jeon, S. Y. Kim, H. C. Kim, T. W. Jang, H. J. Chae, W. J. Choi, M. N. Ha and Y. S. Hong, Ann. Occup. Environ. Med., 2016, 28, 1–8 CrossRef PubMed.
- J. C. Smith, P. V. Allen, M. D. Turner, B. Most, H. L. Fisher and L. L. Hall, Toxicol. Appl. Pharmacol., 1994, 128, 251–256 CrossRef CAS PubMed.
- J. Angerer, L. L. Aylward, S. M. Hays, B. Heinzow and M. Wilhelm, Int. J. Hyg. Environ. Health, 2011, 214, 348–360 CrossRef CAS PubMed.
- R. M. Gardner, M. Kippler, F. Tofail, M. Bottai, J. Hamadani, M. Grander, B. Nermell, B. Palm, K. M. Rasmussen and M. Vahter, Am. J. Epidemiol., 2013, 177, 1356–1367 CrossRef PubMed.
- T. R. Godebo, C. J. Paul, M. A. Jeuland and R. Tekle-Haimanot, Int. J. Hyg. Environ. Health, 2019, 222, 410–418 CrossRef CAS PubMed.
- I. Iavicoli, G. Carelli, C. Lajolo, L. Raffaelli, A. Marinaccio and M. Giuliani, Occup. Med., 2004, 54, 564–566 CrossRef PubMed.
- H. J. Kim, H. S. Lim, K. R. Lee, M. H. Choi, N. M. Kang, C. H. Lee, E. J. Oh and H. K. Park, Int. J. Environ. Res. Public Health, 2017, 14, 1–12 Search PubMed.
- C. L. S. Wiseman, A. Parnia, D. Chakravartty, J. Archbold, R. Copes and D. Cole, Environ. Res., 2019, 169, 261–271 CrossRef CAS PubMed.
- E. McCrudy, Agilent ICP-MS Journal, 2011, 2, 2 Search PubMed.
- C. R. Flores, M. P. Puga, K. Wrobel, M. E. Garay Sevilla and K. Wrobel, Diabetes Res. Clin. Pract., 2011, 91, 333–341 CrossRef CAS PubMed.
- J. P. Goulle, L. Mahieu, J. Castermant, N. Neveu, L. Bonneau, G. Laine, D. Bouige and C. Lacroix, Forensic Sci. Int., 2005, 153, 39–44 CrossRef CAS PubMed.
- P. Schramel, I. Wendler and J. Angerer, Int. Arch. Occup. Environ. Health, 1997, 69, 219–223 CrossRef CAS PubMed.
- C. E. Sieniawska, L. C. Jung, R. Olufadi and V. Walker, Ann. Clin. Biochem., 2012, 49, 341–351 CrossRef CAS PubMed.
- M. Wang, W. Xia, H. Liu, F. Liu, H. Li, H. Chang, J. Sun, W. Liu, X. Sun, Y. Jiang, H. Liu, C. Wu, X. Pan, Y. Li, W. Rang, S. Lu and S. Xu, Toxicol. Res., 2018, 7, 1164–1172 CrossRef CAS PubMed.
- X. Wang, X. Sun, Y. Zhang, M. Chen, G. Dehli Villanger, H. Aase and Y. Xia, Environ. Int., 2020, 139, 1–8 Search PubMed.
- H. L. Zeng, C. W. Liu, J. Lu, X. Wang and L. Cheng, Environ. Sci. Pollut. Res. Int., 2019, 26, 27823–27831 CrossRef CAS PubMed.
- G. Zhang, X. Wang, X. Zhang, Q. Li, S. Xu, L. Huang, Y. Zhang, L. Lin, D. Gao, M. Wu, G. Sun, Y. Song, C. Zhong, X. Yang, L. Hao, H. Yang, L. Yang and N. Yang, Environ. Int., 2019, 123, 164–170 CrossRef CAS PubMed.
- J. Morton, E. Tan, E. Leese and J. Cocker, Toxicol. Lett., 2014, 231, 179–193 CrossRef CAS PubMed.
- B. M. W. Fong, T. S. Siu, J. S. Lee and S. Tam, J. Anal. Toxicol., 2007, 31, 281–287 CrossRef CAS PubMed.
- J. A. Bornhorst, J. W. Hunt, F. M. Urry and G. A. McMillin, Am. J. Clin. Pathol., 2005, 123, 578–583 CrossRef CAS PubMed.
- R. Kalamegham and K. O. Ash, J. Clin. Lab. Anal., 1992, 6, 190–193 CrossRef CAS PubMed.
- M. H. Chan, I. H. Chan, A. P. Kong, R. Osaki, R. C. Cheung, C. S. Ho, G. W. Wong, P. C. Tong, J. C. Chan and C. W. Lam, Pathology, 2009, 41, 467–472 CrossRef CAS PubMed.
- F. Vanhaecke, H. Vanhoe and C. Vandecasteele, Talanta, 1992, 39, 737–742 CrossRef CAS PubMed.
- D. Beauchemin, J. W. McLaren and S. S. Herman, Spectrochim. Acta, Part B, 1987, 42, 467–490 CrossRef.
- J. J. Thompson and R. S. Houk, Appl. Spectrosc., 1987, 41, 801–806 CrossRef CAS.
- T. W. May and R. H. Wiedmayer, Atom. Spectros, 1998, 19, 150–155 CAS.
- N. Yamada, Spectrochim. Acta, Part B, 2015, 110, 31–44 CrossRef CAS.
- P. Borman and D. Elder, in ICH Quality Guidelines: An Implementation Guide, ed. A. Teasdale, D. Elder and R. W. Nims, John Wiley & Sons Inc., New Jersey, 2017, ch. 5, pp. 127–166 Search PubMed.
- A. I. Barros, F. C. Pinheiro, C. D. B. Amaral, R. Lorencatto and J. A. Nobrega, Talanta, 2018, 178, 805–810 CrossRef CAS PubMed.
- S. R. Pappas, Spectroscopy, 2012, 27, 20–31 Search PubMed.
- C. W. Shade and R. J. Hudson, Environ. Sci. Technol., 2005, 39, 4974–4982 CrossRef CAS PubMed.
- Y. Wang and I. D. Brindle, J. Anal. At. Spectrom., 2014, 29, 1904–1911 RSC.
- A. Gonzalez-Antuna, M. Camacho, L. A. Henriquez-Hernandez, L. D. Boada, M. Almeida-Gonzalez, M. Zumbado and O. P. Luzardo, MethodsX, 2017, 4, 328–334 CrossRef PubMed.
- A. Pesch, M. Wilhelm, U. Rostek, N. Schmitz, M. Weishoff-Houben, U. Ranft and H. Idel, J. Expo. Anal. Environ. Epidemiol., 2002, 12, 252–258 CrossRef CAS PubMed.
- D. B. Barr, L. C. Wilder, S. P. Caudill, A. J. Gonzalez, L. L. Needham and J. L. Pirkle, Environ. Health Perspect., 2005, 113, 192–200 CrossRef CAS PubMed.
- M. Lorber, H. M. Koch and J. Angerer, J. Expo. Sci. Environ. Epidemiol., 2011, 21, 576–586 CrossRef CAS PubMed.
- A. Aitio, in Biological Monitoring of Chemical Exposure in the Workplace, ed. T. Solasaari-Pekki and S. Lehtinen, World Health Organization, Geneva, 1996, ch. 2, pp. 20–51 Search PubMed.
- UBA, Bundesgesundheitsblatt - Gesundheitsforsch. - Gesundheitsschutz, 2005, 48, 616–618 CrossRef PubMed.
- C. Schulz, J. Angerer, U. Ewers and M. Kolossa-Gehring, Int. J. Hyg. Environ. Health, 2007, 210, 373–382 CrossRef CAS PubMed.
- C. Schulz, M. Wilhelm, U. Heudorf and M. Kolossa-Gehring, Int. J. Hyg. Environ. Health, 2011, 215, 26–35 CrossRef CAS PubMed.
- UBA, Bundesgesundheitsblatt - Gesundheitsforsch. - Gesundheitsschutz, 2003, 46, 1112–1113 CrossRef.
Footnote |
| † Current address RheinEnergie AG, Parkgürtel 24, 50606, Köln. |
| This journal is © The Royal Society of Chemistry 2023 |