Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-destructive distinction between geogenic and anthropogenic calcite by Raman spectroscopy combined with machine learning workflow†
Sara
Calandra
 *ab,
Claudia
Conti
*ab,
Claudia
Conti
 c,
Irene
Centauro
c,
Irene
Centauro
 a and
Emma
Cantisani
a and
Emma
Cantisani
 d
d
aDepartment of Chemistry Ugo Schiff, University of Florence, 50019 Sesto Fiorentino, Italy. E-mail: sara.calandra@unifi.it
bDepartment of Earth Sciences, University of Florence, 50121 Florence, Italy. E-mail: sara.calandra@unifi.it; irene.centauro@unifi.it
cInstitute of Heritage Science (ISPC), National Research Council (CNR), 20125 Milano, Italy. E-mail: claudia.conti@cnr.it
dInstitute of Heritage Science (ISPC), National Research Council (CNR), 50019 Sesto Fiorentino, Italy. E-mail: emma.cantisani@cnr.it
First published on 30th May 2023
Abstract
Here, we demonstrate, for the first time, the possibility of distinguishing between geogenic and anthropogenic calcite in a non-destructive and effective way. Geogenic calcite derives from natural sedimentary and metamorphic rocks whereas anthropogenic calcite is formed artificially due to the carbonation process in mortars and plaster lime binders. Currently, their distinction is a major unaddressed issue although it is crucial across several fields such as 14C dating of historical mortars to avoid contamination with carbonate aggregates, investigating the origins of pigments, and studying the origins of sediments, to name a few. In this paper, we address this unmet need combining high-resolution micro-Raman spectroscopy with data mining and machine learning methods. This approach provides an effective means of obtaining robust and representative Raman datasets from which samples’ origins can be effectively deduced; moreover, a distinction between sedimentary and metamorphic calcite has been also highlighted. The samples, chemically identical, exhibit systematic and reliable differences in Raman band positions, band shape and intensity, which are likely related to the degree of structural order and polarization effects.
Introduction
Calcite is a mineral widely diffused on the Earth's surface, having different origins. It is mainly present in sedimentary and metamorphic rocks (e.g. marbles) and can also be produced by biological systems and human activities (pyrotechnology origin).Anthropogenic calcite is mainly found as a binder in mortars and plasters and is produced following traditional technologies.1,2 The production of lime mortar is shown in reactions (1)–(3). Air-hardening calcitic limes are obtained by: (1) burning pure limestones at temperatures of 800–950 °C; (2) hydration of calcium oxide; and then, (3) carbonation of calcium hydroxide in air with the formation of calcite.
| CaCO3 + heat = CaO (lime) + CO2 | (1) |
| CaO + H2O = Ca(OH)2 (portlandite) + heat | (2) |
| Ca(OH)2 + CO2 = CaCO3 + H2O. | (3) |
The calcite obtained in this process has the same chemical composition as burnt limestone, but has different textural and mechanical properties. An efficient, fast, effective and widespread technique in laboratories is needed to distinguish calcite from different domains.
Satisfactory results were obtained using Fourier transform infrared spectroscopy (FTIR) in different configurations3–5 and the luminescence properties of calcium carbonate.6,7 Both methods are based on different densities and distributions of atomic defects in the calcite crystal structure.
FTIR can distinguish calcite formed by different processes using the trend lines of anthropogenic and geogenic calcite constructed from the intensity of specific bands.3,4 Luminescence allows us to identify the structural defects in calcite that cause changes in the infrared spectra. The ion substitutions provide luminescence activators or quenchers. Most geogenic forms of CaCO3, e.g. limestone, exhibit red–orange luminescence due to the presence of Mn2+ sites in the calcite crystal lattice.8 In anthropogenic calcite, the luminescence varies, since its formation process involves molecular structure changes, decreasing the number of luminescence centers in the structure.9,10
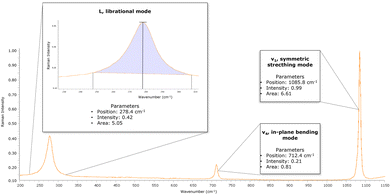
This study aims to verify the feasibility of micro-Raman spectroscopy to distinguish calcite formed by different processes. The Raman spectrum of calcite is characterized by a ν1 sharp band at 1086 cm−1 along with other subsidiary bands at 156 cm−1 (T), 282 cm−1 (L) and 712 cm−1 (ν4).11–17
The Raman technique was used to estimate the cation (Mg2+, Fe2+, and Mn2+) content in carbonate since the vibrational wavenumbers of the translational (T) and librational (L) modes of carbonates are sensitively related to their cation composition,13,17,18 to investigate the changes in atomic bonds in biogenic calcite crystals,19 and to distinguish the degree of crystallinity of calcium carbonate in biological materials,20 evaluating the wavenumbers and the width of v1 and v4 bands.
These papers highlight the suitability of Raman spectroscopy for evidencing the structural and chemical changes that occur in the calcite lattice. Indeed, by studying the variation of the structure of calcite, the short-range order is best detected at the molecular level using Raman spectroscopy.21
The micro-Raman identification of anthropogenic calcite can be used for different purposes: (1) for the selection of the datable fraction from binders in aerial mortars, avoiding any type of contamination with geogenic calcite due to the presence of carbonate aggregates or the remains of underburnt fragments of stone for lime – the accurate 14C dating of mortars is strictly related to the removal of this kind of contaminant;10,22,23 (2) to distinguish the preparation technique of white pigments (crushed rocks or lime-based materials); (3) to identify calcitic wood ash in sediments;3 and (4) to identify the self-healing areas in ancient mortars.24 In these frameworks, since a very small amount of material samples is available, which must be preserved for further analyses, a non-destructive high-resolution micro-Raman technique is recommended and strongly encouraged. In addition, the Raman technique in the portable configuration is more easily applicable than the respective IR spectroscopy (diffuse reflectance spectroscopy) to broaden the use of the calcite identification method in a non-invasive way. It is known that portable FTIR provides spectral modifications, such as distortion, inversion, enhancement, or abatement of infrared bands,25 which can hinder our application.
We selected a wide range of different geogenic and anthropogenic calcites, belonging to different carbonate rocks and mortars. We used high-resolution micro-Raman spectroscopy to accurately measure the order of crystal calcite and identify the information on the spectrum of the geogenic calcite and anthropogenic calcite.
Two technologies, Microsoft Power BI and Python, were used to build a data analysis workflow aiming to distinguish groups of the spectral data acquired for the different calcite samples and to identify their characteristic Raman spectral features. Another objective of the data analysis was to evaluate the accuracy of the identification of geogenic and anthropogenic calcite from spectral data through a comparison between machine learning models.
Materials and methods
Selected samples
The selected samples consist of calcite belonging to Italian geological materials (geogenic calcite samples) and calcite extracted from the binders of air-hardening mortar samples (anthropogenic calcite samples). 13 carbonate rocks, generally burnt to produce quicklime, taken from different Italian quarries and 11 binder mortars collected from historical buildings, factory-made binders and test specimens made in the laboratory were investigated (Table 1). Lumps represent portions of unmixed lime in an aerial mortar produced with traditional technologies.2| ID sample | Material type and provenance | Compositiona | Calcite typeb |
|---|---|---|---|
Cal: calcite; qz: quartz; cl min: clay minerals; portl: portlandite. +++: very abundant; ++: abundant; +: present; *: traces; and −: below the detection limit.a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Via XRPD, SEM-EDS and TGA.b Via XRPD, SEM-EDS and TGA.b ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Via OM, OM-CL, and ATR-FTIR. Via OM, OM-CL, and ATR-FTIR. |
|||
| MAR | Marble, Carrara (Tuscany, Italy) | Cal (+++) | Geogenic |
| CAMP 1 | Marble, Campiglia Marittima (Tuscany, Italy) | Cal (+++) | Geogenic |
| CAMP 2 | Marble, Campiglia Marittima (Tuscany, Italy) | Cal (+++) | Geogenic |
| CAMP 3 | Marble, Campiglia Marittima (Tuscany, Italy) | Cal (+++) | Geogenic |
| MS | Marble, Montagnola Senese (Tuscany, Italy) | Cal (+++) | Geogenic |
| LIM | Marble, Carrara (Tuscany, Italy) | Cal (+++), qz (*) | Geogenic |
| PLEC | Limestone, Pietra di Lecce (Apulia, Italy) | Cal (+++) | Geogenic |
| ALB L | Limestone, Alberese, Monte Morello (Tuscany, Italy) | Cal (+++), cl min (*), qz (*) | Geogenic |
| ALB A | Limestone, Alberese, Monte Morello (Tuscany, Italy) | Cal (+++), cl min (*), qz (*) | Geogenic |
| TRAV | Travertine, Rapolano (Tuscany, Italy) | Cal (+++), qz (*) | Geogenic |
| PGAL | Limestone, Pietra Gallina (Venetian region, Italy) | Cal (+++) | Geogenic |
| PMAT | Limestone, Pietra di Matera (Basilicata, Italy) | Cal (+++) | Geogenic |
| PVIC | Limestone, Pietra di Vicenza (Venetian Region, Italy) | Cal (+++) | Geogenic |
| OS | Ancient plaster, archaeological site | Cal (+++) | Anthropogenic |
| LS01 | Laboratory mortar | Cal (+++), qz (+), portl (*) | Anthropogenic |
| WHL | Factory-made binder | Cal (+++), cl min (*), qz (*) | Anthropogenic |
| CT26L1 | Lime lump, historical building | Cal (+++), qz (++) | Anthropogenic |
| CT26L2 | Lime lump, historical building | Cal (+++), qz (*) | Anthropogenic |
| CT26L4 | Lime lump, historical building | Cal (+++), qz (*) | Anthropogenic |
| CT27L4 | Lime lump, historical building | Cal (+++) | Anthropogenic |
| CT27L1 | Lime lump, historical building | Cal (+++) | Anthropogenic |
| SFC1B1 | Lime binder, historical church | Cal (+++), qz (*) | Anthropogenic |
| SFC1L1 | Lime lump, historical church | Cal (+++), qz (*) | Anthropogenic |
| SFC5B1 | Lime binder, historical church | Cal (+++), qz (*) | Anthropogenic |
In order to systematically investigate the powder, the particle sizes were controlled through different sieves, up to a granulometric class below 25 μm.26
All samples were first analysed through X-ray powder diffractometry (XRPD), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS) and attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and subsequently beamed under a Raman spectrometer. This extensive investigation, not reported here, was performed to control the composition of powders for the suitable selection of those samples consisting mainly of calcite. In fact, the reduction of the number of variables, and the consequent complexity of the system, is essential at this stage for the proper interpretation of spectral changes.
In addition, lime binders and lumps extracted from ancient mortars were thoroughly characterized by optical microscopy (OM), thermogravimetric analysis (TGA) and optical microscopy–cathodoluminescence (OM-CL) imaging to evaluate their reliability for this study (results are not reported here).
Raman spectroscopy
Raman spectra were collected using a high-resolution Renishaw inVia Raman spectrometer coupled to a Leica DMLM microscope. The measurements were carried out with a 785 nm excitation line equipped with a 50× long working distance objective (NA 0.5, a spectral resolution of <1 cm−1 and a theoretical laser spot diameter of 1.9 μm). A laser power of 80 mW and an acquisition time of 5 s per spectrum were used.We decided to focus our attention towards the low-medium region of the spectral range, collected in the range of 100–1400 cm−1. For each powder, we took 10 Raman spectra at slightly different positions.
The wavenumbers, intensities, and areas of typical vibrations of carbonate groups in calcite (L, librational mode; v4, in-plane bending mode; and v1, symmetric stretching mode) were processed with Spectragryph v 1.2.15 software.
The spectra were not baseline corrected but normalized to the v1 height. For each Raman spectrum, from the L, v4, and v1 bands were collected (Fig. 1): (i) the position of the band, to evaluate the wavenumber shift; (ii) the intensity of band, following the method of Chu et al. (2008),3 where the intensity value was subtracted from the specific baseline; and (iii) the area subtended by the band without the baseline.
Given the amount of variables, the extracted parameters were used for statistical analysis of the data to investigate the presence of discriminating factors for distinguishing geogenic and anthropogenic calcite. To better investigate the obtained results, full-width at half-maximums (FWHMs) were recorded for L, v4 and ν1 Raman bands for each sample.
Data exploration and analysis
The proposed workflow integrates Microsoft Power BI data visualization and analysis tool and Python programming language with the Scikit-learn package.27,28 The proposed method involves the following main steps: (1) visual inspection of the dataset; (2) reduction of the dataset dimensionality and segmentation by principal component analysis (PCA) and K-means clustering; and (3) building of machine learning models able to predict the value of the target variable (calcite types) based on the values of the independent variables (logistic regression and random forest models).Raman spectra data are stored in a dataframe: each parameter collected from Raman spectra is called a “feature” (or a “variable”); the 2 possible classes of the target variable are geogenic or anthropogenic calcite. For each variable, outliers are detected and removed by the interquartile range (IQR) method, calculated in Python.29
Then, visual inspection is carried out in Power BI, directly connected to the dataframe, through the key influence factor (KIF) visual, which performs ML.NET SDCA regression implementation.30 According to the second step, PCA was performed in Python, using the sklearn.decomposition.PCA function.31
Before applying the PCA, data are standardized using StandardScaler, a function implemented in the Scikit-learn package, so that all features are at the same scale. From the transformed dataframe after PCA, K-means clustering in Power BI clustering visual is performed. Then, the dataframe is randomly divided into a training set and a testing set (with a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 split ratio) in Python. A comparison between logistic regression and random forest models31–34 is performed in Python, with the Scikit-learn functions logistic regression and random forest classifier, on the PCA components, setting up a repeated K-fold cross-validation with the Scikit-learn function K-fold on the training set, in order to find the best fit to describe the relationship between the target variable and the predictor variables.
30 split ratio) in Python. A comparison between logistic regression and random forest models31–34 is performed in Python, with the Scikit-learn functions logistic regression and random forest classifier, on the PCA components, setting up a repeated K-fold cross-validation with the Scikit-learn function K-fold on the training set, in order to find the best fit to describe the relationship between the target variable and the predictor variables.
Results and discussion
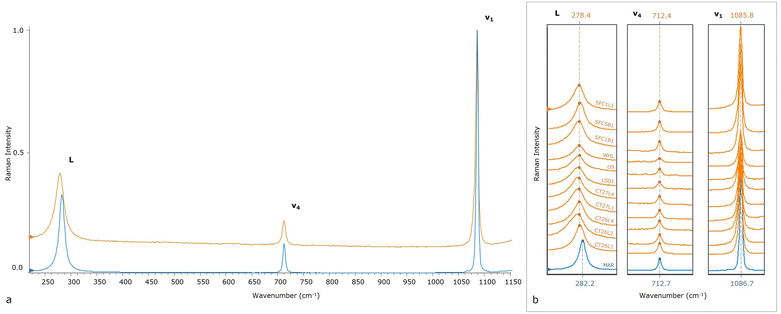
Analytical characterization of the geogenic and anthropogenic calcites
The Raman calcite spectrum (Fig. 1 and 2a) is characterized by an intense band at 1086 cm−1 (v1), along with other subsidiary bands: a weak band at 712 cm−1 (v4) and a medium intensity band at 282 cm−1 (L). These wavenumbers are characteristic of the samples consisting of geogenic calcite, and are not observed in anthropogenic calcite samples, as they exhibit a Raman shift at bands L and v1. Geogenic samples present on average an L varying from 280.4 to 282.4 cm−1, a v4 varying from 712.4 to 713.0 cm−1, and finally a v1 varying from 1086.2 to 1086.9 cm−1 (Table 2). Meanwhile, the anthropogenic samples have an average L ranging from 273.8 to 278.3 cm−1, a v4 rather constant from 712.1 to 712.5 cm−1, and finally a v1 ranging from 1085.4 to 1086.0 cm−1. In Fig. 2b, the marble (blue spectrum) is compared with one of the ten spectra obtained from each anthropogenic sample studied, and a significant variation is highlighted, especially, for L and v1 wavenumbers.| ID sample | L_wavenumber | L_intensity | L_area | v4_wavenumber | v4_intensity | v4_area | v1_wavenumber | v1_intensity | v1_area |
|---|---|---|---|---|---|---|---|---|---|
| MAR | 282.4 | 0.29 | 4.07 | 712.9 | 0.10 | 0.68 | 1086.8 | 0.94 | 4.81 |
| CAMP 1 | 281.9 | 0.29 | 4.38 | 712.5 | 0.09 | 0.54 | 1086.4 | 0.86 | 4.24 |
| CAMP 2 | 280.9 | 0.27 | 4.44 | 712.5 | 0.10 | 0.62 | 1086.5 | 0.91 | 5.01 |
| CAMP 3 | 281.5 | 0.23 | 3.48 | 712.4 | 0.08 | 0.49 | 1086.4 | 0.75 | 3.73 |
| MS | 282.1 | 0.28 | 4.26 | 712.6 | 0.11 | 0.67 | 1086.6 | 0.61 | 4.88 |
| LIM | 280.4 | 0.28 | 5.56 | 712.7 | 0.11 | 0.84 | 1086.4 | 0.96 | 6.71 |
| PLEC | 281.9 | 0.06 | 1.51 | 713.0 | 0.01 | 0.08 | 1086.9 | 0.14 | 0.68 |
| ALB L | 281.4 | 0.05 | 1.53 | 712.8 | 0.01 | 0.09 | 1086.7 | 0.11 | 0.57 |
| ALB A | 281.1 | 0.06 | 1.72 | 712.5 | 0.01 | 0.08 | 1086.5 | 0.19 | 0.78 |
| TRAV | 281.9 | 0.19 | 3.20 | 712.8 | 0.07 | 0.43 | 1086.7 | 0.60 | 3.44 |
| PGAL | 282.0 | 0.09 | 1.73 | 712.7 | 0.02 | 0.14 | 1086.7 | 0.24 | 1.24 |
| PMAT | 282.0 | 0.09 | 1.80 | 712.5 | 0.03 | 0.18 | 1086.6 | 0.28 | 1.47 |
| PVIC | 281.4 | 0.13 | 2.28 | 712.4 | 0.05 | 0.27 | 1086.2 | 0.41 | 2.08 |
| OS | 276.4 | 0.08 | 2.41 | 712.2 | 0.02 | 0.19 | 1085.6 | 0.20 | 1.59 |
| LS01 | 277.6 | 0.09 | 2.11 | 712.3 | 0.03 | 0.30 | 1085.8 | 0.36 | 2.37 |
| WHL | 276.4 | 0.18 | 5.14 | 712.3 | 0.06 | 0.52 | 1085.6 | 0.66 | 5.15 |
| CT26L1 | 275.0 | 0.14 | 4.02 | 712.1 | 0.05 | 0.42 | 1085.4 | 0.46 | 3.36 |
| CT26L2 | 277.4 | 0.21 | 5.34 | 712.4 | 0.08 | 0.58 | 1085.8 | 0.68 | 4.14 |
| CT26L4 | 277.2 | 0.21 | 5.08 | 712.4 | 0.08 | 0.55 | 1085.8 | 0.77 | 5.04 |
| CT27L4 | 273.8 | 0.20 | 6.46 | 712.2 | 0.07 | 0.62 | 1085.4 | 0.62 | 5.11 |
| CT27L1 | 277.8 | 0.25 | 5.99 | 712.5 | 0.09 | 0.70 | 1086.0 | 0.87 | 5.76 |
| SFC1B1 | 277.5 | 0.18 | 4.67 | 712.5 | 0.06 | 0.53 | 1085.9 | 0.62 | 4.89 |
| SFC1L1 | 277.7 | 0.23 | 5.65 | 712.4 | 0.08 | 0.66 | 1085.8 | 0.77 | 5.92 |
| SFC5B1 | 278.3 | 0.24 | 5.42 | 712.5 | 0.09 | 0.69 | 1085.9 | 0.78 | 5.73 |
This systematic discrepancy observed in the Raman shifts of the two calcite groups of different origin prompted us to further investigate the information gathered from the main vibrational modes (in Fig. S1,† 2D plots of the main discriminating parameters are reported and expressed as average values). We determined the wavenumber, intensity, and area of the three main vibrational modes of 24 calcite samples. In Table 2, the parameter average is collected by the spectra. A preliminary observation of results suggests differences between the data gathered.
Data analysis results
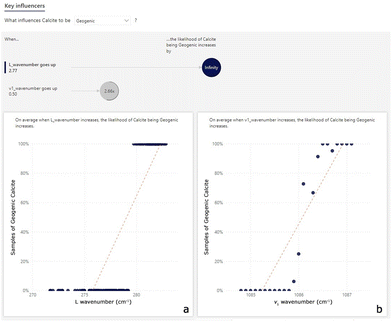
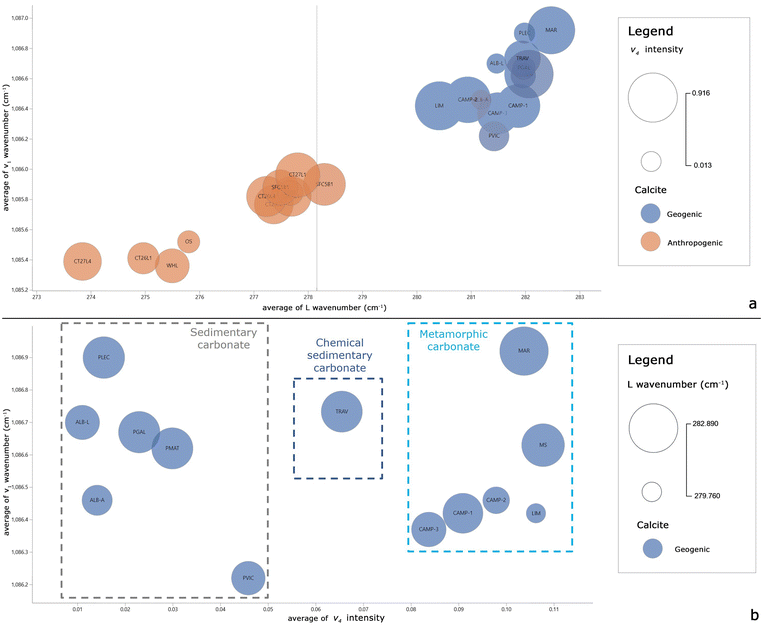
In the first step of the data analysis workflow, visual inspection of the dataset is performed through the key influence factor (KIF) visual. The KIF highlights the L wavenumber and v1 wavenumber as the most important influencers to discriminate geogenic from anthropogenic calcite (Fig. 3). The scatterplot in Fig. 3a shows that all the samples with an L wavenumber value over about 280.0 cm−1 are of geogenic calcite. Similarly, Fig. 3b shows that 85% of samples with a v1 wavenumber value higher than 1086.2 cm−1 consist of geogenic calcites. In addition, another influencing factor could be the v4 intensity: samples with v4 intensity values between 0.026 and 0.098 consist more of anthropogenic calcite than geogenic calcite (Fig. S2†). However, this value is quite variable in geogenic calcite samples, so some fall into this range.From the preliminary KIF results, correlations between the L and v1 wavenumbers and v4 intensity are evaluated. Bubble charts are used to determine whether there is a correlation or a shared trend between at least 3 variables. The L and v1 wavenumbers seem to be the most significant parameters in discriminating geogenic from anthropogenic calcite, thus they are set as the x and y axes in the bubble chart visual in Power BI (Fig. 4).
A different distribution of samples is clearly visible in Fig. 4a, where geogenic samples (in blue) are all located over about 280.0 cm−1 (L wavenumber) and 1086.2 cm−1 (v1 wavenumber). To better highlight the behavior of the v4 intensity of the KIF results, Fig. 4b is built with only geogenic calcite samples and it can be observed that the samples are well separated along the x-axis (v4 intensity). Most of the samples have v4 intensity values below and above the range of 0.026–0.098, in which the majority of anthropogenic calcite samples fall, except for the PMAT, PVIC, TRAV, CAMP 3 and CAMP 1 samples. In particular, the v4 intensity within the geogenic samples is more variable, so this parameter could discriminate against the geogenic calcite types. In addition, the distribution of geogenic samples in the graph could allow a distinction between sedimentary carbonate (ALB A, ALB L, PGAL, PMAT, PLEC, and PVIC) and metamorphic (MAR, CAMP 1, CAMP 2, CAMP 3, LIM, and MS) rocks. Detached from the two groups is the travertine sample (TRAV), since it is a sedimentary rock of chemical origin.
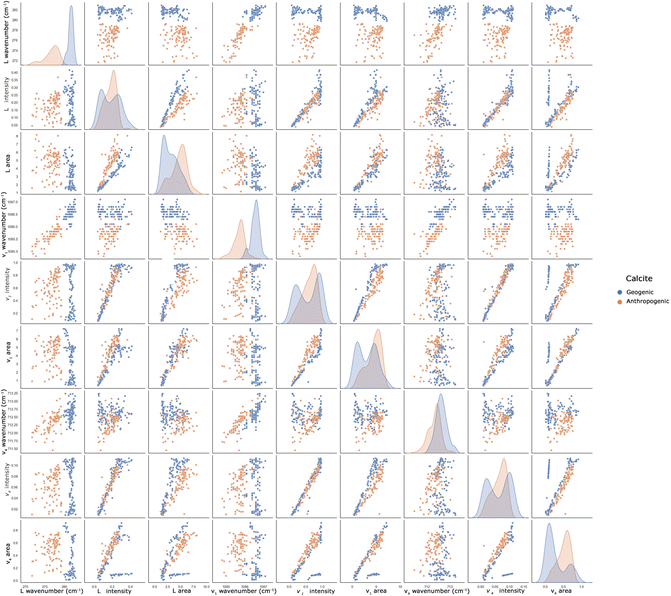
To highlight pairwise relationships between the variables and to complete the visual inspection step in the dataframe, a pairplot is created. Fig. 5 shows that the original dataframe has a high level of multicollinearity, since many variables are strongly correlated with one or more of the other variables. In line with the findings of the KIF analysis, the L and v1 wavenumbers, correlated with all other parameters, allow us to better distinguish the different calcites. The same consideration can be performed for the v4 wavenumber, although a precise discriminating factor cannot be considered.
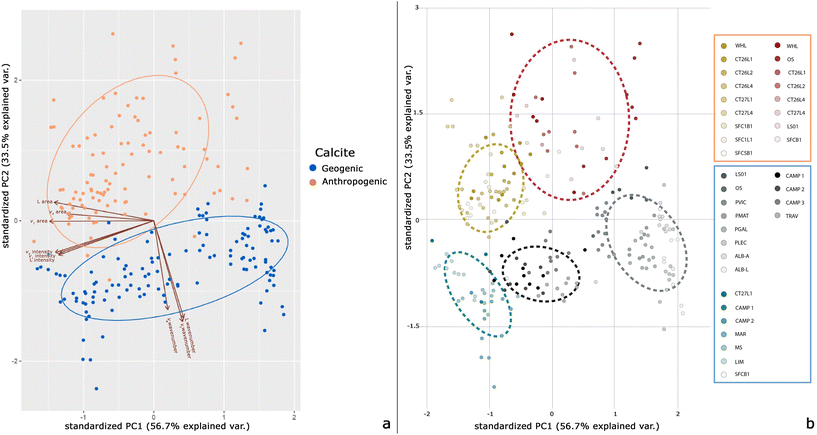
The second step of data analysis provides the PCA in order to eliminate the multicollinearity, reduce the dimensionality of the dataframe and improve the machine-learning algorithm performance. PC1 and PC2 describe 90.2% of the variance (56.7% and 33.5%, respectively). Thus, PCA is performed again keeping only the first 2 PCs. Creating a heatmap of the transformed dataset, it can be seen that no variable is correlated with one or more of the other variables. The Python code is then implemented in Power BI to visualize the biplot of the 2 PCs (Fig. 6). A PCA biplot shows both the PC scores of the samples (dots) and the loadings of the variables (vectors). The PC1 vs. PC2 scores indicate a clear separation of the geogenic from the anthropogenic calcites, except for a few samples (Fig. 6a). The L, v1 and v4 wavenumbers are the most influential variables for calcite distinction and strongly influence the PC2 score.
Another observation on loadings is that the angles between the vectors represent how characteristics correlate with one another: when two vectors are close, forming a small angle, the two variables they represent are positively correlated (e.g., L wavenumber, v1 wavenumber and v4 wavenumber), whereas if they are almost perpendicular, they are not likely to be correlated. As already observed in the KIF analysis and bubble charts, the v4 intensity in the biplot separates geogenic calcites into 3 main groups. From the transformed dataframe after PCA, K-means clustering in Power BI visual is performed (Fig. 6b). K-means is used to identify groups of similar features based on the new representation of data generated by PCA. Of the 5 groups identified, the geogenic calcites are separated into 3 clusters (Fig. 6b), as observed in the bubble chart (Fig. 3b).
PCA and K-means clustering are unsupervised machine learning algorithms that allow us to reduce and segment the data. In order to build a model able to explain the relationship between the target variable (calcite types) and the new variables obtained from PCA, a comparison between supervised machine learning algorithms was performed. Logistic regression classification and random forest classifier algorithms were performed, to extensively investigate the prediction. In general, it is useful to compare different classification or regression models when there are several hypotheses about the relationship between characteristics and the target class and when we want to determine which model provides a better performance for a given classification problem.
The logistic regression algorithm is able to correctly predict 64 out of 67 instances in the test set, resulting in 96% accuracy, while the random forest algorithm is able to correctly predict 62 out of 67 instances, resulting in 93% accuracy (Table 3).
| Validation methods | Calcite | Precision | Recall | F1-score | Support |
|---|---|---|---|---|---|
| Logistic regression | Anthropogenic | 0.95 | 0.97 | 0.96 | 38 |
| Geogenic | 0.96 | 0.93 | 0.95 | 29 | |
| Accuracy | 0.96 | 67 | |||
| Macro avg | 0.96 | 0.95 | 0.95 | 67 | |
| Weighted avg | 0.96 | 0.96 | 0.96 | 67 | |
| Random forest | Anthropogenic | 0.90 | 0.97 | 0.94 | 38 |
| Geogenic | 0.96 | 0.86 | 0.91 | 29 | |
| Accuracy | 0.93 | 67 | |||
| Macro avg | 0.93 | 0.92 | 0.92 | 67 | |
| Weighted avg | 0.93 | 0.93 | 0.92 | 67 | |
As shown in Table 3, the accuracies of the two models are similar, but logistic regression has higher values of precision, recall and F1-score, so it seems to be the best model for explaining the relationship between the target variable and the predictor variables, and to predict the binary outcomes. On the other hand, the performance of the random forest model is relatively robust against parameter specifications and less subject to overfitting, because it depends less on parameter values than other machine learning algorithms such as logistic regression.35
Discussion
The L and v1 wavenumbers are the variables which are more influential for distinguishing calcite domains.These vibrational bands fall into two regions: the L band is due to vibrations of the complete unit cell which are generally referred to as the lattice modes; the v1 band, is caused by the internal modes of the molecular carbonate ion.20,36–38
It is worth noting that Mg is not present in the samples, thus the wavenumber shift is not ascribable to the decrease in the average metal–oxygen bond length (Mg–O bonds are shorter than Ca–O bonds).13,17,39
In order to establish more insights, the FWHMs of L, v4 and v1 bands were measured, and the average values are reported in Table 4. The carbonate rocks are well-crystallized materials, and the average FWHM for the L band is in the range 11.8–17.4 cm−1; for the v4 band, it is in the range 5.1–6.8 cm−1; and for the v1 band, it is in the range 4.3–5.1 cm−1. The binder mortars present significantly higher FWHMs of the L band, in the range 18.1–26.6 cm−1; of v4 in the range 6.3–8.8 cm−1; and of v1 in the range 5.2–6.7 cm−1. It is noteworthy that the more the band positions of anthropogenic calcite are shifted to low values, as in the case of L and ν1 wavenumbers, the higher the FWHM values are.
| L FWHM | v4 FWHM | v1 FWHM | |
|---|---|---|---|
| Geogenic calcite | |||
| MAR | 12.8 | 5.6 | 4.7 |
| CAMP 1 | 11.8 | 5.3 | 4.5 |
| CAMP 2 | 13.4 | 5.6 | 4.9 |
| CAMP 3 | 11.9 | 5.2 | 4.5 |
| MS | 12.6 | 5.4 | 4.6 |
| LIM | 15.9 | 6.8 | 5.1 |
| PLEC | 16.8 | 5.1 | 4.3 |
| ALB L | 13.2 | 5.1 | 4.4 |
| ALB A | 17.4 | 5.8 | 4.8 |
| TRAV | 13.7 | 5.9 | 4.5 |
| PGAL | 13.1 | 5.3 | 4.5 |
| PMAT | 13.3 | 5.6 | 4.4 |
| PVIC | 12.9 | 5.4 | 4.5 |
| Anthropogenic calcite | |||
| OS | 25.3 | 8.7 | 6.7 |
| LS01 | 22.2 | 8.8 | 5.2 |
| WHL | 21.9 | 7.5 | 6.3 |
| CT26L1 | 24.3 | 7.2 | 5.9 |
| CT26L2 | 20.2 | 6.5 | 5.3 |
| CT26L4 | 19.3 | 6.6 | 5.4 |
| CT27L4 | 26.6 | 7.5 | 6.4 |
| CT27L1 | 18.1 | 6.3 | 5.2 |
| SFC1B1 | 21.4 | 7.6 | 6.0 |
| SFC1L1 | 20.6 | 7.6 | 6.2 |
| SFC5B1 | 18.5 | 7.0 | 5.5 |
The relatively large FWHMs reflect the Raman spectroscopic features of a structural disorder in calcite crystals or a small crystalline order: the broader the spectra bandwidth, the lower the degree of mineral crystallinity. This disorder changes the selection rules of the Raman active modes: more phonon modes become Raman active, and each phonon mode broadens its features.40 The slope of the phonon dispersion curves of the vibrational modes determines the shift of the bands:41 a negative slope results in a shift towards lower wavenumbers. As a consequence, calcite disordered systems reflect a systematic larger FWHM and a shift of certain modes toward lower wavenumbers when compared with the crystalline counterparts, as already observed in the literature also for other minerals.20,42,43
Crystallinity can vary for many reasons, mainly regarding the increase in crystal defects or in amorphous or nanocrystalline phases in the sample.44 During the carbonation process, the degree of structural order of calcite can be compromised by several factors, i.e. the time of setting and environmental conditions, and there is a general agreement in the literature on the possibility of achieving complete carbonation.45 The findings achieved in this study highlight that the carbonation process leads to the formation of structurally disordered calcite crystals.
The further outcome of the present study concerns the discriminating power of v4 intensity within the geogenic samples: sedimentary carbonates, travertine and metamorphic rocks are distinguishable by following their v4 intensity. The average calcite crystal size is higher in geogenic samples than in anthropogenic samples, and thus polarization effects in Raman spectra of rocks should be considered. The crystallographic orientation of calcite with respect to the incident light polarization is one of the key factors responsible for the relative intensity ratio change of bands in calcite Raman spectra. Interestingly, the outcomes of the present study highlight that mineral orientation depends on the growth and deformation processes of crystals during diagenesis and Raman spectroscopy can be used to distinguish the preferred mineral orientation.46 The polarization effect is negligible in anthropogenic calcite due to the reduced crystal size, as confirmed by the quite homogeneous v4 intensity.
Conclusions
In this work, for the first time, the distinction between geogenic and anthropogenic calcite was made using high-resolution micro-Raman spectroscopy in a non-destructive way. The observed systematic Raman shifts in L and v1 bands for anthropogenic calcite prompted us to apply data analysis and integrated machine learning methods. The successful parameters (among the position of the band, the intensity of the band, the area subtended by the bands and the FWHMs of L, v4, and v1) for distinguishing the calcite origins were identified from KIF, PCA, K-means clustering, and the relationship between the target and the predictor variables using the logistic regression and random forest models.The proposed method was shown to be effective in discriminating anthropogenic calcite in pyrotechnological materials (i.e. mortars and plasters) in order to select the most suitable carbonate fraction for radiocarbon dating purposes. The application of this approach could be extended to the evaluation of the carbonate origins of pigments and sediments in archaeological contexts. Furthermore, types of carbonate rocks are distinguishable on the basis of the v4 intensity, paving the way to other geological and petrographic applications. The measurements could also be potentially performed in situ using portable Raman instruments with a suitable spectral resolution.
The structurally ordered–disordered, crystallinity degree and the polarization effect are the main factors that influence the Raman spectral signature of calcite. These findings encourage further investigations, i.e. with single crystal X-ray diffraction to obtain detailed information about the crystal structure of the different examined calcites, extending this research also to the precipitation of secondary calcite in different fields.
Author contributions
Calandra S.: methodology, experiments and data collection, data analysis, writing, review and editing. Conti C.: methodology, data collection and acquisition, and writing and review. Centauro I.: data analysis, system design and development, and writing; Cantisani E.: methodology, project planning, and writing and review.Conflicts of interest
There are no conflicts to declare.References
- G. Artioli, M. Secco and A. Addis, in The Contribution of Mineralogy to Cultural Heritage, EMU Notes Miner, 2019, vol. 20, pp. 151–202. DOI:10.1180/EMU-notes.20.4.
- E. Cantisani, F. Fratini and E. Pecchioni, Minerals, 2022, 12(1), 41, DOI:10.3390/min12010041.
- V. Chu, L. Regev, S. Weiner and E. Boaretto, J. Archaeol. Sci., 2008, 35(4), 905–911, DOI:10.1016/j.jas.2007.06.024.
- L. Regev, K. M. Poduska, L. Addadi, S. Weiner and E. Boaretto, J. Archaeol. Sci., 2010, 37(12), 3022–3029, DOI:10.1016/j.jas.2010.06.027.
- S. Calandra, E. Cantisani, B. Salvadori, S. Barone, L. Liccioli, M. Fedi and C. A. Garzonio, J. Phys.: Conf. Ser., 2022, 2204(1), 012048, DOI:10.1088/1742-6596/2204/1/012048.
- H. G. Machel, R. A. Mason, A. N. Mariano and A. Mucci, in Luminescence Microscopy and Spectroscopy: Qualitative and Quantitative Applications, Soc. Sedim. Geol., 1991, vol. 25, pp. 37–57. DOI:10.2110/scn.91.25.0009.
- A. El Ali, V. Barbin, G. Calas, B. Cervelle, K. Ramseyer and J. Bouroulec, Chem. Geol., 1993, 104(1–4), 189–202, DOI:10.1016/0009-2541(93)90150-H.
- D. K. Richter, T. Götte, J. Götze and R. D. Neuser, Mineral. Petrol., 2003, 79, 127–166, DOI:10.1007/s00710-003-0237-4.
- M. B. Toffolo, G. Ricci, L. Caneve and I. Kaplan-Ashiri, Sci. Rep., 2019, 9(1), 16170, DOI:10.1038/s41598-019-52587-7.
- M. B. Toffolo, G. Ricci, R. Chapoulie, L. Caneve and I. Kaplan-Ashiri, Radiocarbon, 2020, 62(3), 545–564, DOI:10.1017/RDC.2020.21.
- R. S. Krishnan, Proc. Indiana Acad. Sci., 1945, A22, 182–193 CrossRef.
- D. Krishnamurti, Proc. Indiana Acad. Sci., 1957, A46, 183–202 CrossRef.
- W. D. Bischoff, S. K. Sharma and F. T. MacKenzie, Am. Mineral., 1985, 70(5–6), 581–589 CAS.
- I. P. Herman and F. Magnotta, J. Appl. Phys., 1987, 61(11), 5118–5128, DOI:10.1063/1.338286.
- K. E. Kuebler, A. Wang, K. Abbott and L. A. Haskin, Lunar and Planetary Science XXXII, 2001, p. 1889 Search PubMed.
- M. De La Pierre, C. Carteret, L. Maschio, E. André, R. Orlando and R. Dovesi, Chem. Phys., 2014, 140(16), 164509, DOI:10.1063/1.4871900.
- L. Borromeo, U. Zimmermann, S. Andò, G. Coletti, D. Bersani, D. Basso and E. Garzanti, J. Raman Spectrosc., 2017, 48(7), 983–992, DOI:10.1002/jrs.5156.
- S. H. Urashima, M. Morita, S. Komatani and H. Yui, Anal. Chim. Acta, 2023, 340798, DOI:10.1016/j.aca.2023.340798.
- E. Zolotoyabko, E. N. Caspi, J. S. Fieramosca, R. B. Von Dreele, F. Marin, G. Mor and Y. Politi, Cryst. Growth Des., 2010, 10(3), 1207–1214, DOI:10.1021/cg901195t.
- U. Wehrmeister, D. E. Jacob, A. L. Soldati, N. Loges, T. Häger and W. Hofmeister, J. Raman Spectrosc., 2011, 42(5), 926–935, DOI:10.1002/jrs.2835.
- C. Giacovazzo, Fundamentals of Crystallography, International Union of Crystallography, Oxford Univ., Press, 3rd edn, 2011 Search PubMed.
- P. Urbanová, E. Boaretto and G. Artioli, Radiocarbon, 2020, 62(3), 503–525, DOI:10.1017/RDC.2020.43.
- A. Lindroos, Å. Ringbom, J. Heinemeier, I. Hajdas and J. Olsen, Radiocarbon, 2020, 62(3), 565–577, DOI:10.1017/RDC.2020.5.
- L. M. Seymour, J. Maragh, P. Sabatini, M. Di Tommaso, J. C. Weaver and A. Masic, Sci. Adv., 2023, 9(1), eadd1602, DOI:10.1126/sciadv.add1602.
- Z. M. Khoshhesab, in Infrared spectroscopy-Materials science, engineering and technology, IntechOpen, 2012, pp. 233–244. DOI:10.5772/2055.
- C. Indelicato, I. Osticioli, J. Agresti, D. Ciofini, A. Mencaglia, M. Perotti and S. Siano, Eur. Phys. J. Plus, 2022, 137(3), 359, DOI:10.1140/epjp/s13360-022-02536-7.
- J. M. Palma-Ruiz, A. Torres-Toukoumidis, S. E. González-Moreno and H. G. Valles-Baca, Heliyon, 2022, e08959, DOI:10.1016/j.heliyon.2022.e08959.
- J. Hao and T. K. Ho, J. Educ. Behav. Stat., 2019, 44(3), 348–361, DOI:10.3102/1076998619832248.
- H. Vinutha, B. Poornima and B. Sagar, in Information and Decision Sciences. Advances in Intelligent Systems and Computing, ed. S. Satapathy, J. Tavares, V. Bhateja and J. Mohanty, Springer, Singapore, 2018, p. 701. DOI:10.1007/978-981-10-7563-6_53.
- F. K. Sufi, Software Impacts, 2022, 11, 100218, DOI:10.1016/j.simpa.2022.100218.
- C. Zhu, C. U. Idemudia and W. Feng, Inform. Med. Unlocked, 2019, 17, 100179, DOI:10.1016/j.imu.2019.100179.
- X. Fan, W. Ming, H. Zeng, Z. Zhang and H. Lu, Analyst, 2019, 144(5), 1789–1798, 10.1039/C8AN02212G.
- A. Amjad, R. Ullah, S. Khan, M. Bilal and A. Khan, Vib. Spectrosc., 2018, 99, 124–129, DOI:10.1016/j.vibspec.2018.09.003.
- Y. Liu, Y. Wang and J. Zhang, in Information Computing and Applications. ICICA 2012. Lecture Notes in Computer Science, ed. B. Liu, M. Ma and J. Chang, Springer, Berlin, Heidelberg, 2012, vol. 7473, DOI:10.1007/978-3-642-34062-8_32.
- R. Couronné, P. Probst and A. L. Boulesteix, BMC Bioinf., 2018, 19, 270, DOI:10.1186/s12859-018-2264-5.
- K. Nakamoto, J. Fujita, S. Tanaka and M. Kobayashi, J. Am. Chem. Soc., 1957, 79(18), 4904–4908 CrossRef CAS.
- W. B. White, in The Infrared Spectra of Minerals, Mineralogical Society of Great Britain and Ireland, The Carbonate Minerals, 1974, vol. 4, pp. 227–284. DOI:10.1180/mono-4.12.
- B. E. Scheetz and W. B. White, Am. Mineral., 1977, 62, 36–50 CAS.
- D. Wang, L. M. Hamm, R. J. Bodnar and P. M. Dove, J. Raman Spectrosc., 2012, 43(4), 543–548, DOI:10.1002/jrs.3057.
- Q. Wang, D. D. Allred and L. V. Knight, J. Raman Spectrosc., 1995, 26(12), 1039–1043, DOI:10.1002/jrs.1250261204.
- C. L. Jiang, W. Zeng, F. S. Liu, B. Tang and Q. J. Liu, J. Phys. Chem. Solids, 2019, 131, 1–9, DOI:10.1016/j.jpcs.2019.03.011.
- B. Xu and K. M. Poduska, Phys. Chem. Chem. Phys., 2014, 16(33), 17634–17639, 10.1039/c4cp01772b.
- J. Perrin, D. Vielzeuf, D. Laporte, A. Ricolleau, G. R. Rossman and N. Floquet, Am. Mineral., 2016, 101(11), 2525–2538, DOI:10.2138/am-2016-5714.
- R. F. Perez and J. Martinez-Frias, J. Raman Spectrosc., 2003, 34(5), 367–370, DOI:10.1002/jrs.1003.
- J. I. Alvarez, R. Veiga, S. Martínez-Ramírez, M. Secco, P. Faria, P. N. Maravelaki and J. Válek, Mater. Struct., 2021, 54(2), 63, DOI:10.1617/s11527-021-01648-3.
- A. E. Murphy, R. S. Jakubek, A. Steele, M. D. Fries and M. Glamoclija, J. Raman Spectrosc., 2021, 52(6), 1155–1166, DOI:10.1002/jrs.609.
Footnote |
| † Electronic supplementary information (ESI) available: SFig. 1: Figure depicting the 2D plots of v1, v4, L wavenumbers are reported and expressed as average values; SFig. 2: Scatterplot from the key influence factor visual: increase in % of anthropogenic calcite samples of v4 intensity values. For more details about data analysis, see DOI: https://doi.org/10.1039/d3an00441d |
| This journal is © The Royal Society of Chemistry 2023 |