Tetrabutylammonium-chloride-glycerol of deep eutectic solvent functionalized MnO2: a novel mimic enzyme for the quantitative and qualitative colorimetric detection of L-cysteine
Jing
Chen
 ab,
Hangdao
Qin
ab,
Hangdao
Qin
 a,
Lu
Xu
a,
Lu
Xu
 ab,
Senlin
Leng
a and
Jun
Chang
*a
ab,
Senlin
Leng
a and
Jun
Chang
*a
aCollege of Material and Chemical Engineering, Tongren University, Tongren 554300, P.R. China. E-mail: junchang85@163.com
bTongren Key Laboratory for Modernization Research, Development and Utilization of Traditional Chinese Medicine and National Medicine, Tongren University, Tongren 554300, PR China
First published on 29th November 2022
Abstract
L-Cysteine is a common amino acid that plays an important role in human livelihood and production. Therefore, a novel method for the simultaneous quantitative and qualitative determination of L-cysteine by a colorimetric detection system is proposed. As a viable oxidase mimic, [N4444]Cl-G/MnO2, which consisted of MnO2 nanosheets functionalized by a tetrabutylammonium chloride–glycerol ([N4444]Cl-G) based deep eutectic solvent (DES) was fabricated. Owing to the oxidation of MnO2 nanosheets, [N4444]Cl-G/MnO2 could oxidize the colorless 3,3′,5,5′-tetramethylbenzidine (TMB) into a blue product (oxTMB) with the characteristic UV–vis spectrum absorbance at 652 nm. The oxidation of TMB by DES/MnO2 was inhibited when L-cysteine was introduced, and the absorbance decreased proportionally with the increase in L-cysteine concentration. Due to this inhibition effect, a colorimetric detection system ([N4444]Cl-G/MnO2-TMB) was developed for the quantitative determination of L-cysteine. Under optimal conditions, the assay showed good linearity over the concentration range of 0.125–2.00 μg mL−1 with a low detection limit of 5.96 ng mL−1. A study of the inhibition mechanism demonstrated that the sulfhydryl group of L-cysteine could decompose [N4444]Cl-G/MnO2 into Mn2+, thus limiting the conversion of TMB to oxTMB. In addition, the [N4444]Cl-G/MnO2-TMB system was used in test strips for the visual qualitative detection of L-cysteine. The selectivity and test strip results demonstrated the high selectivity, simple operation, and rapid response of the [N4444]Cl-G/MnO2-TMB system for the qualitative detection of L-cysteine. Given the satisfying performance of the detection strategy, colorimetric sensing based on the [N4444]Cl-G/MnO2-TMB system is considered to have prospective application value in the quantitative and qualitative detection of L-cysteine.
Introduction
As a common amino acid, L-cysteine is the main substance of nails, toenails, skin and hair and also plays a critical role in human livelihood and production. In particular, as an essential intracellular biothiol, L-cysteine has been used as a critical parameter for diagnosing various diseases, such as hair depigmentation, skin lesions, edema, liver damage, brain damage, Alzheimer's, and a retarded growth rate.1–4 Due to the biological activity of L-cysteine, the accurate quantitation and fast monitoring of L-cysteine are extremely important for clinical medicine.5 Generally, several methods have been applied to detect L-cysteine, mainly including fluorescence/mass spectroscopy, high-performance liquid chromatography, and electrochemical techniques.6 However, these methods have not been used extensively because of some limitations, such as complicated material synthesis, long operation time, and the need for complex and expensive instruments. Thus, establishing new methods for the high-performance quantitative or qualitative analysis of L-cysteine is crucial.In recent years, due to the advantages of its simple operation, there is no need for expensive instruments, visible detection by the naked eye, and the nanozyme-based colorimetric method has gradually attracted increasing attention and has been widely used in the field of analysis and detection.7 To date, research on the reported oxidase mimics as colorimetric materials has mainly focused on metal nanoclusters/nanoparticles,8,9 metal–organic frameworks,10 graphene oxide,11 iron oxides,12 carbon dots,13etc. MnO2 nanosheet, a type of transition metal oxide, has low toxicity and good biocompatibility, rich redox chemistry, and excellent catalytic and oxidase-like activity. Owing to its superior chemical and physical properties, many researchers have combined MnO2 nanosheets with chem/biosensing techniques for the determination of dopamine,14 hydroquinone,15 glutathione,16 salmonella,17etc. More importantly, different from the reported peroxidase mimics for colorimetric sensing, MnO2 nanosheets represent a label-free colorimetric probe that can oxidize the colorless 3,3′,5,5′-tetramethylbenzidine (TMB) into a blue product (oxTMB), which has a characteristic UV–vis spectrum absorbance at 652 nm, without the addition of H2O2. Nevertheless, it is worth noting that bare MnO2 nanosheets can easily agglomerate and have poor dispersibility in water. Thus, some necessary functionalization modifications need to be taken to enhance the dispersibility and oxidase-like activity of MnO2 nanosheets.
Deep eutectic solvent (DES), as an emerging solvent, has been identified as offering new possibilities to replace traditional solvents in the field of organic and material synthesis,18,19 separation,20 electrochemistry,21 and so on. DES is made of hydrogen-bond acceptors (HBAs) and hydrogen-bond donors (HBDs) in a proper ratio under certain conditions.22 Generally, choline chloride, betaine, quaternary ammonium/phosphonium salts, and amino acid are mainly considered as HBAs, while glycerol, ethylene glycol, polymer, carbohydrates, carboxylic acids, and urea are regard as HBDs.23–25 Its unique composition can be tuned to design the DES to obtain specific properties, so it is often used as a functional monomer to functionalize various nanomaterials, for example metal–organic frameworks,19 graphene oxide,26 silica microspheres,27 carbon dots.28 From the above reported literature, it is known that the properties of nanomaterials can be promoted and improves by the introduction of DES.
Inspired by the above, we tried to modify DES as a functional monomer on MnO2 nanosheets to prepare DES/MnO2. By combining their physical and chemical properties, the poor dispersion of MnO2 nanosheets in water could be overcome, and the enzyme-like property of DES/MnO2 could be further improved. To the best of our knowledge, there are no reported studies on the simultaneous quantitative and qualitative detection of L-cysteine. Herein, we aimed to design a sensitive, convenient, and label-free colorimetric strategy for the visual detection of L-cysteine a through DES/MnO2-TMB sensing system (as shown in Scheme 1). First, a DES of tetrabutylammonium chloride–glycerol ([N4444]Cl-G) was chosen as the functional monomer to be modified on MnO2 nanosheets to synthesize the novel mimic enzyme [N4444]Cl-G/MnO2. Then, we combined this with 3,3′,5,5′-tetramethylbenzidine (TMB) to construct a scheme for quantitative and qualitative colorimetric detection of L-cysteine based on the [N4444]Cl-G/MnO2-TMB system, respectively. At the end, the detection mechanisms and test strips study are also investigated and discussed.
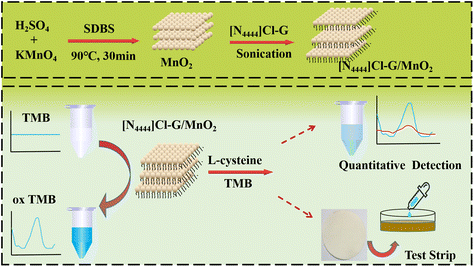 | ||
| Scheme 1 Preparation of [N4444]Cl-G/MnO2 and its application for the colorimetric detection of L-cysteine. | ||
Experimental section
Materials and apparatus
L-Arginine (Arg, ≥98%), L-proline (Pro, ≥99%), L-phenylalanine (Phe, 99%), L-aspartic acid (Asp, 99%), glutathione (GHS, 99%), and L(+)-ascorbic acid (AA, 99%) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). D-(+)-Glucose (Glu, >99%), glycerol (>99%), ethanol (EtOH, ≥99%), potassium chloride (KCl, 99%), sodium chloride (NaCl, ≥99%), glutamic acid (Glu, ≥98%), L-lysine (Lysine, ≥98%), glycine (Gly, >99%), L-alanine (Ala, ≥98.5%), L-sodium dodecyl benzene sulfonate (SDBS, ≥95%), sulfuric acid, and KMnO4 were bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Valine (Val, 98%), L-serine (Ser, 99%), 3,3′,5,5′-tetramethylbenzidine (TMB, >98%), and tetrabutylammonium chloride ([N4444]Cl>95%) were supplied by Adamas Reagent Co., Ltd (Shanghai, China). Homocysteine (Hcy, 97%) was obtained from Sigma-Aldrich Chemical Reagent Co., Ltd (Anhui, China). All the reagents used were of analytical grade.The instruments used for the characterizations and experiments were: an ESCALAB 250Xi X-ray photoelectron spectroscopy system (XPS, Thermo Scientific, U.S.); FT-IR spectrometry system (PerkinElmer, U.S.); STA 409 thermal gravimetric analyzer (TGA, Netzsch, Germany); INOVA 400NB NMR (Varian, Japan); D/Max 2500 X-ray diffraction system (XRD, Rigaku, Japan); QYC200 incubator shaker (Shanghai, China); UV2450 UV–vis spectrophotometry system (Shimadzu, Japan); Zetasizer Nano-ZS90 dynamic light scattering system (DLS, Malvern, Britain); and JEM-3010 transmission electron microscopy system (TEM, JEOL, Japan).
Synthesis of [N4444]Cl-G, MnO2 nanosheet and [N4444]Cl-G/MnO2
The detailed process for synthesizing MnO2 nanosheets, [N4444]Cl-G, and [N4444]Cl-G/MnO2 can be found in the reported literature29 and in our previous research.30Fig. 1 shows the synthetic route for the [N4444]Cl-G. The prepared DES was characterized by 1H NMR, 13C NMR, and FT-IR (as also can be seen in our previous research30). The synthesis of [N4444]Cl-G/MnO2 can be briefly described as follows: 1 mL of [N4444]Cl-G and 4 mL of methanol were put into a 50 mL round-bottom flask, and then MnO2 nanosheets (100 mg) were added to the above mixed solution. Following ultrasonic reaction for 2 h and then washing with ethanol 2 times, finally the crude [N4444]Cl-G/MnO2 was obtained. The crude product was centrifuged and then freeze-dried to obtain the final [N4444]Cl-G/MnO2, which was then dispersed in water and prepared as a stock solution with a concentration of 0.1 mg mL−1 for further use in the study.Preparation of the quantitative colorimetric system
First, 150 μL of [N4444]Cl-G/MnO2 stock solution and 1650 μL of sodium acetate buffer (HAc-NaAc solution, 0.1M) were added into a 2 mL centrifuge tube. Then, 100 μL of L-cysteine with different concentrations was selected as the model analyte and added into the above centrifuge tube, and incubated 1 h at the appropriate temperature. Subsequently, 100 μL of TMB dissolved in ethanol (1 mg mL−1) was added into the above centrifuge tube and continuously incubated for 1 h. Finally, the UV–vis spectrum absorbance of the solution was measured because the [N4444]Cl-G/MnO2 could directly catalyze the oxidation of TMB (colorless) to oxTMB (blue) with a characteristic spectrum at λmax = 652 nm.Results and discussion
Characterization of [N4444]Cl-G/MnO2
According to the literature31 and Fig. 2A(a), two characteristic peaks were found at 641.19 and 653.38 eV that could be assigned to Mn 2p3/2 and Mn 2p1/2 of MnO2, respectively. As shown in Fig. 2A(b), the high-resolution XPS spectrum of N 1s easily confirmed that the [N4444]Cl-G was modified on the MnO2 nanosheets. In addition, Fig. 2A(c) presents the XPS full survey of [N4444]Cl-G/MnO2, where distinct peaks of C 1s, N 1s, and O 1s could be clearly observed at binding energies of 284.86, 402.04, and 529.19 eV, respectively. All the XPS spectrum results verified that [N4444]Cl-G/MnO2 had been successfully synthesized. Fig. 2B shows the FT-IR spectra of MnO2 and [N4444]Cl-G/MnO2. As shown by the curve of MnO2, the characteristic peak at 516 cm−1 referred to νMn–O and νMn–O–Mn.32 After the MnO2 nanosheet was modified by [N4444]Cl-G, several distinct peaks of [N4444]Cl-G could be clearly observed from the line of [N4444]Cl-G/MnO2. For example, the broad band at 3350 cm−1 was assigned to the νO–H of [N4444]Cl-G. The other characteristic peaks at 2962, 2973, and 2875 cm−1 could be attributed to νC–H of CH3 and CH2. Comparing the FT-IR spectra of MnO2 and [N4444]Cl-G/MnO2, it could be definitively proven that the [N4444]Cl-G had been correctly modified on the surface of MnO2 nanosheet. TGA analysis was performed to explore the weight percentage of [N4444]Cl-G on the surface of the MnO2 nanosheet. As shown in Fig. 2C, when the temperature exceeded 250 °C, the [N4444]Cl-G started to decompose, with a weight loss of about 9.84%, which indicated the grafting yield of [N4444]Cl-G modified on the surface of the MnO2 nanosheet was approximately 9.84 wt%. As can be seen from Fig. 2D, the crystallographic structure of [N4444]Cl-G/MnO2 was researched by powder XRD. Four remarkable diffraction peaks were observed at 2θ = 12.48°, 18.54°, 36.14°, and 65.54°, which indicated the characteristic XRD pattern of MnO2, which was consistent with previous studies.33,34 TEM was performed to analyze the microscopic structure of [N4444]Cl-G/MnO2. As shown in Fig. 2E and its inset, [N4444]Cl-G/MnO2 had good dispersibility in water and the average lateral dimension was about 50 nm.Optimization of the colorimetric detection platform
The single-factor optimization method was adopted to establish the optimum [N4444]Cl-G/MnO2-TMB catalytic platform. The incubation pH, temperature, time, and the concentration of [N4444]Cl-G/MnO2 solution were systematically explored. In this section, all the sample solutions had their UV–vis spectrum absorbance recorded at λmax = 652 nm measured after the incubation reaction. Also, all the analysis detection conditions were carried out at room temperature without specific instructions. First, a series of HAc–NaAc buffer solutions (0.1 M, pH value from 3.0 to 6.0), incubation temperatures (from 10 °C to 45 °C), and times (from 0 to 120 min) were tested one by one. As shown in Fig. 3A–C, the test results clearly showed that the maximum catalytic ability was obtained at pH 4.0, with a temperature around 25 °C, and incubation time of approximately 60 min. Next, in order to get the best amount of [N4444]Cl-G/MnO2, a series of concentrations of [N4444]Cl-G/MnO2 solution (100 μL) were dropped in to a mixture solution containing 100 μL of TMB (1.0 mg mL−1) and 1800 μL of HAc-NaAc solution (pH 4.0, 0.1 M). As seen from Fig. 3D, the UV–vis spectrometer absorbance changed in response to the [N4444]Cl-G/MnO2 concentrations. The absorption intensity almost kept linearly in a rising tendency when the [N4444]Cl-G/MnO2 concentration was increased from 0 to 20 μL mL−1, then once the concentration exceeded 20 μL mL−1, the absorption intensity curve began to decline. Therefore, the optimal conditions for the colorimetric detection platform were determined as follows: pH 4.0, room temperature, incubation time of 1 h, and concentration of [N4444]Cl-G/MnO2 of 20 μL mL−1, respectively.Under the above optimal conditions, this study also systematically investigated the change in the UV–vis absorbance values at λmax = 652 nm of MnO2, [N4444]Cl-G/MnO2, and TMB solution when the three substances were placed alone for 1 h, respectively. As shown in Table 1, the UV–vis absorption values of the three substances showed no significant change under the continuous monitoring time. In particular, the UV–vis absorbance value of TMB solution was always kept under 0.003, almost the same as the background noise value of the instrument, and therefore it could be completely ignored. In comparing the UV–vis absorbance changes in Fig. 3C and Table 1, one could effectively ignore the influence of the changes of the UV–vis absorbance for three types of substances during the incubation time.
| Sample | Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 1 (min) | 10 (min) | 20 (min) | 30 (min) | 40 (min) | 50 (min) | 60 (min) | |
| MnO2 alone (20 μL mL−1) | 0.015 | 0.012 | 0.013 | 0.012 | 0.014 | 0.012 | 0.012 |
| [N4444]Cl-G/MnO2 alone (20 μL mL−1) | 0.006 | 0.008 | 0.009 | 0.008 | 0.008 | 0.007 | 0.006 |
| TMB alone (50 μg mL−1) | 0.003 | 0.001 | 0.001 | 0.002 | 0.003 | 0.001 | 0.001 |
Quantitative colorimetric detection
The [N4444]Cl-G/MnO2-TMB system was applied for the colorimetric quantification of L-cysteine. All the test conditions were carried out under the optimal conditions as follows: pH 4.0, room temperature, incubation time of 1 h, and concentration of [N4444]Cl-G/MnO2 of 20 μL mL−1, respectively. Then, a series of different concentrations of L-cysteine were successively added to the above conditions for reaction, and the UV–vis absorbance value at λmax = 652 nm was recorded. As illustrated in Fig. 4A, at the beginning, with the continuous addition of L-cysteine, the value of ΔA (A0 − A) showed a linear and rapid growth pattern. Then, as the amount of L-cysteine continued to increase, the growth rate of ΔA gradually slowed down. A good relationship between ΔA and L-cysteine concentration could be obtained with the concentration of L-cysteine increasing from 0.125 to 2.00 μg mL−1. The regression equation was determined as y = 0.3361x − 0.0202 (R2 = 0.996) and the LOD (S/N = 3) was 5.96 ng mL−1 (as seen from Fig. 4B). Previous literature35 showed that the normal concentration of L-cysteine in healthy serum was 152.8–266.5 nmol mL−1. Hence, through unit conversion, one could get the linear detection interval of L-cysteine concentrations as ranging from 1.03 to 16.5 nmol mL−1. These result showed that this method could be used when the L-cysteine concentration was much lower than the normal concentration of L-cysteine in healthy serum. In addition, Fig. 4C shows the transformation of the UV–vis absorbance spectra.Moreover, in order to highlight the different catalytic activities of [N4444]Cl-G/MnO2, and MnO2 nanosheet, four concentrations of L-cysteine (in the linear detection interval) were selected to react with the [N4444]Cl-G/MnO2 and MnO2 nanosheets, respectively. All the detection conditions were optimal, and only the concentration of L-cysteine was changed. The results from the tests are listed in Table 2. It could be clearly seen that the catalytic activity of the MnO2 nanosheets was slightly higher than that of [N4444]Cl-G/MnO2, but not too obvious. On the contrary, the ΔA error value (±) and RSD value of [N4444]Cl-G/MnO2 were much smaller than those of the MnO2 nanosheets, which indicated that the catalytic activity of [N4444]Cl-G/MnO2 was more stable and precise after the functional modification of [N4444]Cl-G. This might be due to the large steric hindrance of [N4444]Cl-G, which could effectively prevent the agglomeration of MnO2 nanosheets and allow [N4444]Cl-G/MnO2 to have better dispersion.
| L-Cysteine | Sample | ΔA | Average of ΔA | ΔA err(±) | RSD (%) | |||
|---|---|---|---|---|---|---|---|---|
| 0.50 (μg mL−1) | MnO2 | Sample number | 1 | 2 | 3 | — | — | — |
| ΔA | 0.1293 | 0.1623 | 0.215 | 0.1689 | 0.04339 | 25.677 | ||
| [N4444]Cl-G/MnO2 | Sample number | 1 | 2 | 3 | — | — | — | |
| ΔA | 0.1463 | 0.1443 | 0.1473 | 0.1460 | 0.00153 | 1.046 | ||
| 1.00 (μg mL−1) | MnO2 | Sample number | 1 | 2 | 3 | — | — | — |
| ΔA | 0.3283 | 0.3003 | 0.4203 | 0.3496 | 0.06278 | 17.956 | ||
| [N4444]Cl-G/MnO2 | Sample number | 1 | 2 | 3 | — | — | — | |
| ΔA | 0.3183 | 0.3123 | 0.3133 | 0.31463 | 0.00321 | 1.022 | ||
| 1.50 (μg mL−1) | MnO2 | Sample number | 1 | 2 | 3 | |||
| ΔA | 0.6153 | 0.5673 | 0.4413 | 0.5413 | 0.08987 | 16.602 | ||
| [N4444]Cl-G/MnO2 | Sample number | 1 | 2 | 3 | ||||
| ΔA | 0.4953 | 0.4873 | 0.5013 | 0.4946 | 0.00702 | 1.420 | ||
| 2.00 (μg mL−1) | MnO2 | Sample number | 1 | 2 | 3 | — | — | — |
| ΔA | 0.6153 | 0.5673 | 0.4413 | 0.6086 | 0.03940 | 6.473 | ||
| [N4444]Cl-G/MnO2 | Sample number | 1 | 2 | 3 | — | — | — | |
| ΔA | 0.5983 | 0.5953 | 0.5903 | 0.5946 | 0.00404 | 0.6796 | ||
Mechanism of L-cysteine detection
Owing to the sulfhydryl group of L-cysteine, [N4444]Cl-G/MnO2 was reduced to produce Mn2+ by L-cysteine.29 Due to the decomposition of [N4444]Cl-G/MnO2, the conversion of TMB to oxTMB was inhibited, resulting in decreased absorbance in the UV–vis spectrum. A mechanism study was conducted under the optimal detection conditions with 100 μL (50 μg mL−1) L-cysteine. Fig. 5(A) and (C) depict the corresponding TEM images of the [N4444]Cl-G/MnO2-TMB system for L-cysteine detection before and after incubating for 1 h. Furthermore, in order to exclude the possibility of self-decomposition, a TME image of individual [N4444]Cl-G/MnO2 left to stand for 1 h was obtained (as seen in Fig. 5B). Comparing the microscopic morphologies of [N4444]Cl-G/MnO2 in Fig. 5A and B, it could be clearly seen that the micro morphology and size of [N4444]Cl-G/MnO2 had not changed significantly after 1 h of standing, which could effectively eliminate an impact from the degradation of [N4444]Cl-G/MnO2. As seen from Fig. 5A and C, it could be found that the [N4444]Cl-G/MnO2 had been destroyed with no obvious aggregates, which further proved that [N4444]Cl-G/MnO2 had decomposed.Selectivity
In order to evaluate the specificity of the [N4444]Cl-G/MnO2-TMB system for L-cysteine detection, several interfering substances were tested. The selectivity tests were carried out with a working solution with 150 μL of [N4444]Cl-G/MnO2, 1650 μL of HAc-NaAc (pH 4.0) and 100 μL of TMB. Next, 100 μL (50 μg mL−1) of common amino acids, including Pro, Lysine, Ala, Asp, Val, Gly, Arg, Phe, Glu, and Ser, were used as interfering substances, respectively. As seen from Fig. 6A, no significant changes in the absorption intensity were observed except for L-cysteine. The absorbance was obviously lower in the presence of L-cysteine in contrast to the other amino acids, including Pro, Lysine, Ala, Asp, Val, Gly, Arg, Phe, Glu, and Ser. These results clearly illustrated that the proposed method could provide good selectivity to detect L-cysteine from common amino acids. Besides, three kinds of amino acids with reducing properties or interfering substances containing a thiol group (such as Hcy, GSH, AA) were tested as possible interfering substances. Here, 100 μL (50 μg mL−1) of Hcy, GSH, and AA were added to the above working solution, respectively. As shown within the red dashed box in Fig. 6, Hcy, GSH, and AA all had a great impact on the [N4444]Cl-G/MnO2-TMB detection system. This might be because these 3 substances all could decompose the MnO2 nanosheets into Mn2+ ions,36,37 which was consistent with the detection mechanism of L-cysteine. Apparently, this is a common disadvantage of colorimetric detection methods, and similar results can be seen in the published literature.38–40 To eliminate the effects of the above three kinds of interfering substances, corresponding measures should be adopted, for example, by adding masking agents41 or they should be further separated by combining with other precision techniques (such as high-performance liquid chromatography), etc. Therefore, this is also an opportunity and challenge for colorimetric detection analysis methods. | ||
| Fig. 6 Absorbance intensity signal and corresponding photographs of the [N4444]Cl-G/MnO2-TMB system for L-cysteine and common analytes, respectively. | ||
Detection by test strips
Because of the easy storage, low cost, simple operation, and quick test operation of paper-based sensors, test strip recognition was developed to pave a new way for performing L-cysteine analysis. The indicating test paper was simply fabricated as follows: first, the well-dispersed [N4444]Cl-G/MnO2-TMB solution (1.0 mg mL−1) was prepared and poured into a surface dish. Then a piece of Whatman qualitative filter paper was soaked in the above solution. A few minutes later, the paper was taken out and dried in the air. As shown in Fig. 7, the indicating test paper displayed a pale yellow color under daylight. A capillary tube was used to absorb the TMB solution as ink and then used to write “AB” on the test paper. The ink immediately showed up with a blue color and displayed good stability. Nevertheless, for the L-cysteine test strip study, a mixed solution composed of 1.0 mg mL−1 TMB and 50 μg mL−1 of analyte solution (including Pro, Lysine, Ala, Asp, Val, Gly, Arg, Phe, Glu, and Ser, respectively) was dropped on to the indicating test paper. The color change of the test strip could be observed with the naked eye. One could vividly see that the tested amino acids caused an evident blue color signal on the indicating test paper, except for L-cysteine. In addition, the L-cysteine and various amino acids analyte solutions were also dropped on the test paper as the control groups. As shown by the black dashed line figures in Fig. 7, the color of L-cysteine and various amino acids analyte solution in the test strip without [N4444]Cl-G/MnO2-TMB were transparent and there was no obvious color change after drying. All the test strip results proved that the [N4444]Cl-G/MnO2-based test strip had a rapid response, simple operation, and portable features. More importantly, the [N4444]Cl-G/MnO2-based test strip has potential as a highly promising platform for the convenient detection of L-cysteine.Conclusions
In conclusion, a simple and convenient colorimetric method was developed for the facile sensing of L-cysteine based on [N4444]Cl-G-functionalized MnO2 nanosheets with TMB. The [N4444]Cl-G selected in this study could effectively prevent the agglomeration of MnO2 nanosheets through its large steric hindrance effect, thus further enhancing the dispersion and catalytic activity of [N4444]Cl-G/MnO2 in water. Most importantly, due to the special role of MnO2 nanosheets, the presented colorimetric method was label-free and did not require hydrogen peroxide. The colorimetric system was then successfully utilized for fabricating a paper-based device for L-cysteine's detection. In summary, the [N4444]Cl-G/MnO2-TMB platform holds promising potential applications in sensing for the quantitative and qualitative colorimetric detection of L-cysteine.Author contributions
Jing Chen: conceptualization, methodology, investigation, writing – original draft. Hangdao Qin: data curation and validation. Lu Xu: formal analysis and software. Senlin Leng: investigation and methodology. Jun Chang: writing – review & editing, resources.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors greatly appreciate the financial supports by the National Natural Science Foundation of China (No. 51864042, 22166031), Department of Education of Guizhou Province, China (QJJ[2022]092, QJJ[2022]003), Guizhou Science and Technology Department (No. QKHPTRC[2020]5009, QKHZC[2020]4Y043), Tongren Science and Technology Bureau (No.TSKY2019-3), the Research Fund for the Doctoral Program of Tongren University (No. trxyDH2107).References
- A. Aziz, M. Asif, G. Ashraf, T. Iftikhar, M. Ajmal, H. Liu and S. Wang, Chem. Eng. J., 2022, 440, 135985 CrossRef.
- P. M. Getsy, S. M. Baby, R. B. Gruber, B. Gaston, T. H. J. Lewis, A. Grossfield, J. M. Seckler, Y.-H. Hsieh, J. N. Bates and S. J. Lewis, Front. Pharmacol., 2022, 13, 1755 Search PubMed.
- C. Kalinke, P. R. de Oliveira, B. C. Janegitz and J. A. Bonacin, Sens. Actuators, B, 2022, 362, 191797 CrossRef.
- M. Zan, C. Li, D. Zhu, L. Rao, Q.-F. Meng, B. Chen, W. Xie, X. Qie, L. Li, X. Zeng, Y. Li, W.-f. Dong and W. Liu, J. Mater. Chem. B, 2020, 8, 919–927 RSC.
- B. Zhang, H. Zhang, M. Zhong, S. Wang, Q. Xu, D.-H. Cho and H. Qiu, Chin. Chem. Lett., 2020, 31, 133–135 CrossRef CAS.
- J. Chen, S. Hu, Y. Cai, X. Liu, Y. Wu, Y. Dai and Z. Wang, Analyst, 2022, 147, 915–922 RSC.
- R. Li, X. He, R. Javed, J. Cai, H. Cao, X. Liu, Q. Chen, D. Ye and H. Zhao, Sci. Total Environ., 2022, 834, 155428 CrossRef CAS.
- Y.-C. Gao, C. Wang, C.-X. Zhang, H.-W. Li and Y. Wu, J. Mater. Sci. Technol., 2022, 109, 140–146 CrossRef.
- Z. Zhu, L. Gong, X. Miao, C. Chen and S. Su, Biosensors, 2022, 12(5), 260 CrossRef CAS.
- X. Lai, Y. Shen, S. Gao, Y. Chen, Y. Cui, D. Ning, X. Ji, Z. Liu and L. Wang, Biosens. Bioelectron., 2022, 213, 114446 CrossRef CAS.
- H. Tian, J. Liu, J. Guo, L. Cao and J. He, Talanta, 2022, 242, 123320 CrossRef CAS.
- C. Zhou, J. Chen, G. Wang and X. Su, Microchim. Acta, 2022, 189, 135 CrossRef CAS PubMed.
- X. Xu, Q. Sun, Y. Ma, X. Jiang, N. Niu and L. Chen, Sens. Actuators, B, 2022, 364, 131881 CrossRef CAS.
- Y. Xing, M. Chen, Y. Zhao, J. Xu and X. Hou, Microchim. Acta, 2022, 189, 12 CrossRef CAS.
- Y. Ma, M. Zhu, Q. He, M. Zhao and H. Cui, ACS Sustainable Chem. Eng., 2022, 10(17), 5651–5658 CrossRef.
- Y. Liu, J. Yan, Z. Sun, Y. Huang, X. Li and Y. Jin, Microchim. Acta, 2022, 189(4), 1–10 CrossRef.
- L. Xue, N. Jin, R. Guo, S. Wang, W. Qi, Y. Liu, Y. Li and J. Lin, ACS Sens., 2021, 6, 2883–2892 CrossRef PubMed.
- L. Chen, Y. Xiong, H. Qin and Z. Qi, ChemSusChem, 2022, 15(13), e202102635 CrossRef PubMed.
- X. Zhang, P. Zhu, Q. Li and H. Xia, Front. Chem., 2022, 10, 911674 CrossRef PubMed.
- L. B. Santos, R. S. Assis, J. A. Barreto, M. A. Bezerra, C. G. Novaes and V. A. Lemos, TrAC, Trends Anal. Chem., 2022, 146, 116478 CrossRef.
- S. Azmi, M. F. Koudahi and E. Frackowiak, Energy Environ. Sci., 2022, 15, 1156–1171 RSC.
- J. Chen, Y. Wang, S. Leng, L. Xu and Z. Xie, Talanta, 2022, 248, 123566 CrossRef PubMed.
- L. B. Santos, R. S. Assis, J. A. Barreto, M. A. Bezerra, C. G. Novaes and V. A. Lemos, TrAC, Trends Anal. Chem., 2022, 146, 116478 CrossRef.
- V. Andruch, R. Halko, J. Tuček and J. Płotka-Wasylka, TrAC, Trends Anal. Chem., 2022, 147, 116510 CrossRef.
- A. A. A. Mutalib and N. F. Jaafar, J. Environ. Chem. Eng., 2022, 10, 107422 CrossRef.
- N. Mehrabi, H. Lin and N. Aich, Chem. Eng. J., 2021, 412, 128577 CrossRef.
- R. Shi, B. Zhang, W. Chen, X. Lan, Y. Yang and T. Mu, J. Colloid Interface Sci., 2021, 604, 635–642 CrossRef PubMed.
- M. Wang, X. Kang, L. Deng, M. Wang, Z. Xia and D. Gao, Food Chem., 2021, 345, 128817 CrossRef.
- X. Yan, Y. Song, X. Wu, C. Zhu, X. Su, D. Du and Y. Lin, Nanoscale, 2017, 9, 2317–2323 RSC.
- J. Chen, Y. Wang, X. Wei, R. Ni, J. Meng, F. Xu and Z. Liu, Mikrochim. Acta, 2019, 187, 1–7 Search PubMed.
- Q. Wang, H. Pang, Y. Dong, Y. Chi and F. Fu, Mikrochim. Acta, 2018, 185, 291 CrossRef PubMed.
- X. Wang, D. Wang, Y. Guo, C. Yang, X. Liu, A. Iqbal, W. Liu, W. Qin, D. Yan and H. Guo, Biosens. Bioelectron., 2016, 77, 299–305 CrossRef PubMed.
- L. Qi, Z. Yan, Y. Huo, X. M. Hai and Z. Q. Zhang, Biosens. Bioelectron., 2017, 87, 566–571 CrossRef PubMed.
- J. Liu, L. Meng, Z. Fei, P. J. Dyson, X. Jing and X. Liu, Biosens. Bioelectron., 2017, 90, 69–74 CrossRef PubMed.
- Z. Huang, Y. Yang, Y. Long and H. Zheng, Anal. Methods, 2018, 10, 2676–2680 RSC.
- D. Wang, Y.-t. Meng, Y. Zhang, Q. Wang, W.-j. Lu, S.-m. Shuang and C. Dong, Sens. Actuators, B, 2022, 367, 132135 CrossRef.
- Q.-Y. Cai, J. Li, J. Ge, L. Zhang, Y.-L. Hu, Z.-H. Li and L.-B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef PubMed.
- Q. Yang, L. Li, F. Zhao, Y. Wang, Z. Ye and X. Guo, Mater. Lett., 2019, 248, 89–92 CrossRef.
- H. Xue, M. Yu, K. He, Y. Liu, Y. Cao, Y. Shui, J. Li, M. Farooq and L. Wang, Anal. Chim. Acta, 2020, 1127, 39–48 CrossRef PubMed.
- M. Zhong, M. Chi, F. Ma, Y. Zhu, C. Wang and X. Lu, ACS Sustainable Chem. Eng., 2018, 6, 16482–16492 CrossRef.
- W. Na, N. Li and S. Xingguang, Sens. Actuators, B, 2018, 274, 172–179 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |






