Open Access Article
Open Access ArticleA mild synthetic strategy for removing acetic acid from fast pyrolysis-derived bio-oils utilizing Friedel–Crafts acylation reactions†
Han Byeol
Kim‡
 ab,
Pratip Kumar
Dutta‡
a,
Duck-Hyung
Lee
ab,
Pratip Kumar
Dutta‡
a,
Duck-Hyung
Lee
 b and
Seo-Jung
Han
b and
Seo-Jung
Han
 *ac
*ac
aChemical & Biological Integrative Research Center, Korea Institute of Science and Technology, 5 Hwarang-ro 14-gil, Seongbuk-gu, Seoul 02792, Republic of Korea. E-mail: sjhan@kist.re.kr
bDepartment of Chemistry, Sogang University, 35 Baekbeom Ro, Seoul 04107, Republic of Korea
cDivision of Bio-Medical Science & Technology, KIST School, University of Science and Technology, 5 Hwarang-ro 14-gil, Seongbuk-gu, Seoul 02792, Republic of Korea
First published on 19th October 2022
Abstract
Fast pyrolysis-derived bio-oils contain numerous oxygenated components, including acetic acid and formic acid. However, these acids present in fast pyrolysis-derived bio-oils are responsible for the instability and corrosiveness of the oils. Although these acids have been removed from fast pyrolysis-derived bio-oils by esterification with alcohols to form acetates, these acetates could not be transformed to compounds with a suitable number of carbon atoms required to be used as fuels. In this work, Friedel–Crafts acylation reactions were conducted to remove acetic acid to form acetophenones, which possess more than eight carbons and could be converted to valuable components of fuels.
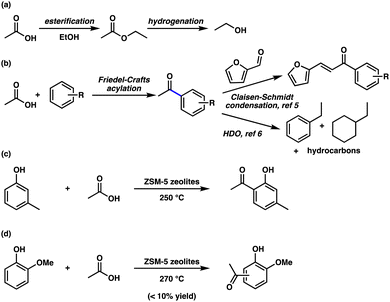
Bio-oils produced by the fast pyrolysis of biomass have been considered potential substitutes for conventional fuels. However, direct utilization of crude fast pyrolysis-derived bio-oils is limited because they contain high quantities of water (15–30 wt%) and oxygen (30–40 wt%) from oxygenated species (e.g., acids, aldehydes, alcohols, and sugars).1 The high degree of oxygenated species in crude fast pyrolysis-derived bio-oils results in chemical instability, corrosiveness, and low heating values. Thus, upgrading processes are required for bio-oils obtained from fast pyrolysis to be used as fuels.
The extreme instability and corrosiveness of bio-oils obtained from fast pyrolysis have been attributed to the pH levels of 2–3 resulting from the significant amount of carboxylic acids, mainly acetic acid and formic acid. The acidity of fast pyrolysis-derived bio-oils leads to the corrosion of construction materials (e.g., carbon steel and aluminum) and sealing materials. In addition, secondary condensation or polymerization of reactive components, such as aldehydes, ketones, and phenols, in the presence of acids changes the physicochemical properties of fast pyrolysis-derived bio-oils.2,3
Thus, significant efforts have been made to develop strategies for the esterification of acids with alcohols, which can also be applicable from fast pyrolysis-derived bio-oils, to lower the acidity and improve the stability of fast pyrolysis-derived bio-oils (Scheme 1a).1,4 However, esterification of acids and subsequent hydrogenation produce alcohols, which do not contain the required number of carbon atoms to be used as components for replacing conventional fuels. Here, a mild and efficient synthetic strategy to convert acetic acid into valuable components containing more than eight carbons, which could be used as substitutes for conventional oils, was developed (Scheme 1b). Insertion of the acetyl group from acetic acid on aromatic components in fast pyrolysis bio-oils by Friedel–Crafts acylation reactions provided acetophenones. The resulting acetophenones from Friedel–Crafts acylation reactions can be converted into valuable components using further upgrading techniques5,6 An array of C-acetylated products were prepared from both phenolic and non-phenolic compounds. Although similar strategies have been investigated recently, these reactions require high temperatures, and only the m-cresol or guaiacol substrate, with which poor yields of C-acylated products were observed, was examined.7–9
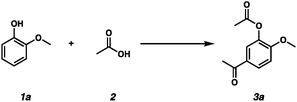
We began our Friedel–Crafts acylation reaction studies with guaiacol as a bio-oil model compound because various methoxyphenol substrates constitute almost 20% of the bio-oil mixture.10 Initially, we attempted the Friedel–Crafts acylation reaction using commercially available and inexpensive P2O5 (Table 1, entries 1–7).11 An investigation of different solvents revealed that the neat conditions were optimal (Table 1, entries 1–3). We discovered that O-acylation proceeded as well as the Friedel–Crafts acylation reaction to generate 3a. The use of 1.0 equivalent of P2O5 provided the desired product in 54% yield (Table 1, entry 4). Acetylated compound 3a was obtained at 140 °C in 61% yield, although a slightly diminished yield was obtained at 150 °C (Table 1, entries 5 and 6). The use of 2.0 equivalents of P2O5 produced product 3a in 61% yield (Table 1, entry 7). However, the use of graphene oxide (GO) for activating both acetic acid and aryl groups12 or Lewis acidic Nb2O513,14 instead of P2O5 led to lower yields (Table 1, entries 8–16).
| Entry | Conditions | Solvent | Time (h) | T (°C) | AcOH (equiv.) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Yield of isolated product. | ||||||
| 1 | P2O5 (0.5 equiv.) | DCE | 12 | 120 | 2.0 | 0 |
| 2 | P2O5 (0.5 equiv.) | DMF | 12 | 120 | 2.0 | 0 |
| 3 | P2O5 (0.5 equiv.) | Neat | 12 | 120 | 15 | 43 |
| 4 | P2O5 (1.0 equiv.) | Neat | 12 | 120 | 15 | 54 |
| 5 | P2O5 (1.0 equiv.) | Neat | 12 | 140 | 15 | 61 |
| 6 | P2O5 (1.0 equiv.) | Neat | 12 | 150 | 10 | 58 |
| 7 | P2O5 (2.0 equiv.) | Neat | 12 | 140 | 10 | 61 |
| 8 | GO (100 wt%) | Neat | 12 | 120 | 10 | 30 |
| 9 | GO (100 wt%) | CH3NO2 | 12 | 120 | 10 | 0 |
| 10 | GO (100 wt%) | CHCl3 | 12 | 120 | 10 | Trace |
| 11 | GO (100 wt%) | 1,4-Dioxane | 12 | 120 | 10 | 0 |
| 12 | GO (100 wt%) | Triethylene glycol | 12 | 120 | 10 | 0 |
| 13 | GO (100 wt%) | IPA | 12 | 120 | 10 | 25 |
| 14 | Nb2O5 (1.0 equiv.) | Neat | 12 | 120 | 10 | 0 |
| 15 | Nb2O5 (1.0 equiv.) | MeNO2 | 12 | 120 | 10 | 25 |
| 16 | Nb2O5 (1.0 equiv.) | DMSO | 12 | 120 | 10 | Trace |
With the optimized reaction conditions in hand, we investigated the scope of phenolic substrates found in bio-oils for the Friedel–Crafts acylation transformation (Scheme 2). ortho-Alkyl substituted phenols, o-cresol 1b, 2-ethylphenol 1c, and 2-propylphenol 1d, produced the corresponding products in moderate yields (Scheme 2, entries 1–3). In addition, alkyl substituents at the meta position were tolerated under the reaction conditions (Scheme 2, entries 4 and 5). para-Cresol 1g, 4-ethylphenol 1h, and 4-propylphenol 1i generated the desired products in 56%, 40%, and 48% yields, respectively (Scheme 2, entries 6–8). However, 2,6-dimethoxyphenol 1j provided acetylated product 3j in a reduced yield (Scheme 2, entry 9). 2-Methoxy-4-methylphenol 1k and 3,4-dimethylphenol 1l produced the desired products in 57% and 53% yields, respectively (Scheme 2, entries 10 and 11).
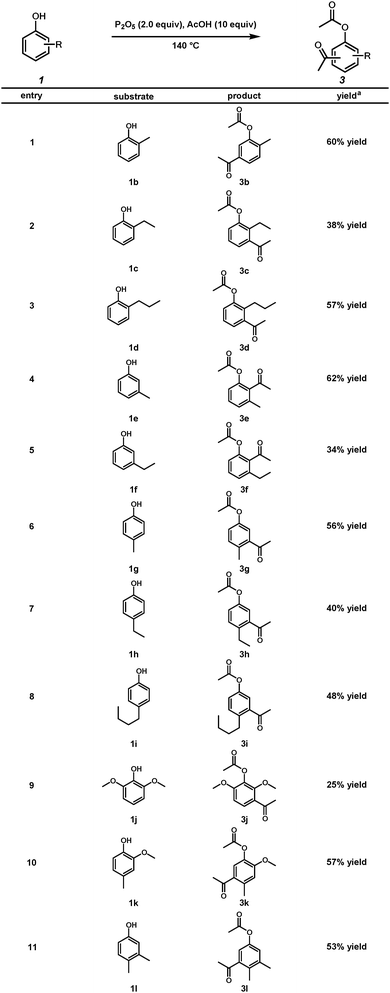 | ||
| Scheme 2 Friedel–Crafts acylation on phenolic components of fast pyrolysis-derived bio-oils (aYield of the isolated product). | ||
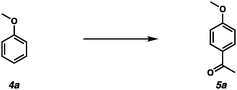
Non-phenolic aromatic components are also commonly found in bio-oils. Therefore, the Friedel–Crafts acylation reaction on non-phenolic aromatic components was optimized using anisole 4a as a model substrate (Table 2). Initially, we attempted the Friedel–Crafts acylation reaction under optimized conditions for phenolic substrates. However, desired compound 5a was obtained in a diminished yield (Table 2, entry 1). The use of more equivalents of acetic acid provided 5a in higher yields (Table 2, entries 2–4). 4-Methoxyacetophenone 5a was obtained in 70% yield using 20 equivalents of acetic acid (Table 2, entry 4). We discovered that the addition of 1.0 equivalent of P2O5 resulted in a lower yield (Table 2, entry 5).
We investigated the substrate scope of non-phenolic components in bio-oils (Scheme 3). 2-Methylanisole 4b produced acetophenone 5b in 53% yield (Scheme 3, entry 1). Xylene 4c was tolerated under the reaction conditions, producing 5c in a moderate yield (Scheme 3, entry 2). In addition, Friedel–Crafts acylation of indan 4d and furan 4e generated 5d and 5e, respectively (Scheme 3, entries 3 and 4).
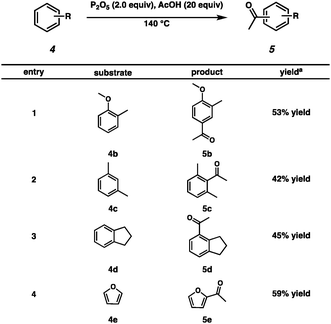 | ||
| Scheme 3 Friedel–Crafts acylation on non-phenolic components of fast pyrolysis-derived bio-oils (aYield of the isolated product). | ||
With the scope of the established Friedel–Crafts acylation reactions, we sought to demonstrate the utility of the synthesized acylated products in the synthesis of valuable components containing more than eight carbons, which could be used as substitutes for the conventional oil after further upgrading. Claisen–Schmidt condensation of furfural 6 and acylated products from non-phenolic components of fast pyrolysis-derived bio-oils 5a and 5e produced desired products 7 and 8, respectively in good yields under mild reaction conditions (Scheme 4). In addition, Claisen–Schmidt condensation of furfural 6 and 3a that was synthesized from phenolic components of fast pyrolysis-derived bio-oils and acetic acid generated 9 in moderate yield (Scheme 4).
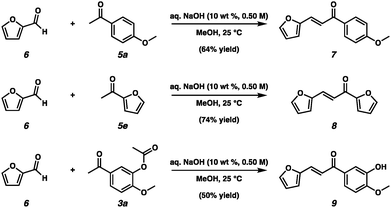 | ||
| Scheme 4 Claisen–Schmidt Condensation between furfural 6 and acetylated compounds from the Friedel–Crafts acylation reactions. | ||
In summary, the Friedel–Crafts acylation reaction was utilized to remove acetic acid to upgrade bio-oils. The resulting acetophenones can be converted into more valuable components for fuels. Friedel–Crafts acylation using 2 equivalents of P2O5 provided the corresponding acetophenones in moderate to good yields. Both the phenolic and non-phenolic components of the bio-oils were all well tolerated under our reaction conditions. In addition, we found that the products from the Friedel–Crafts acylation reactions can be further upgraded by Claisen–Schmidt condensation under mild reaction conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the KIST Institutional Program (2E31624, 2E31690, 2V09235, 2E31629, and 2E3162D), and the Technology Development Program to Solve Climate Change of the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT (NRF-2020M1A2A2079798). This research was also supported by the National Research Council of Science & Technology (NST), granted by the Korean government (MSIT) (No. CPS21061-100). Additionally, this research was supported by Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN22C0063000022, Republic of Korea).References
- N. Lohitharn and B. H. Shanks, Catal. Commun., 2009, 11, 96 CrossRef CAS; J. Chen, Q. Cai, L. Lu, F. Leng and S. Wang, ACS Sustainable Chem. Eng., 2017, 5, 1073 CrossRef.
- C. Liu, H. Wang, A. M. Karin, J. Sun and Y. Wang, Chem. Soc. Rev., 2014, 43, 7594 RSC.
- S. Czernik and A. V. Bridgwater, Energy Fuels, 2004, 18, 590 CrossRef CAS; S. Czernik, D. K. Johnson and S. Black, Biomass Bioenergy, 1994, 7, 187 CrossRef.
- Q. Zhang, L. Zhang, T. Wang, Y. Xu, Q. Zhang, L. Ma, M. He and K. Li, Energy Procedia, 2014, 61, 1033 CrossRef CAS; J.-J. Wang, J. Chang and J. Fan, Energy Fuels, 2010, 24, 3251 CrossRef; Y. Liu, Z. Li, J. J. Leahy and W. Kwapinski, Energy Fuels, 2015, 29, 3691 CrossRef; Y. Xu, L. Zhang, W. Lv, C. Wang, C. Wang, X. Zhang, Q. Zhang and L. Ma, Catalysts, 2021, 11, 818 CrossRef; L. Ciddor, J. A. Bennett, J. A. Hunns, K. Wilson and A. F. Lee, J. Chem. Technol. Biotechnol., 2015, 90, 780 CrossRef.
- G. Yadav and A. R. Yadav, RSC Adv., 2014, 4, 63772 RSC.
- C. González, P. Marín, F. V. Díez and S. Ordóñez, Energy Fuels, 2015, 29, 8208 CrossRef.
- H. K. Chau, D. E. Resasco, P. Do and S. P. Crossley, J. Catal., 2022, 406, 48 CrossRef CAS.
- S. Gutiérrez-Rubio, M. Shamzhy, J. Cejka, D. P. Serrano, I. Moreno and J. M. Coronado, Appl. Catal., B, 2021, 285, 119826 CrossRef.
- M. K. Montañez-Valencia, C. L. Padró and M. E. Sad, Appl. Catal., B, 2020, 278, 119317 CrossRef.
- I. B. Adilina, N. Rinaldi, S. P. Simanungkalit, F. Aulia, F. Oemry, G. B. G. Stenning, I. P. Silverwood and S. F. Parker, J. Phys. Chem. C, 2019, 123, 21429 CrossRef CAS.
- (a) A. Zarei, A. R. Hajipour and L. Khazdooz, Tetrahedron Lett., 2008, 49, 6715 CrossRef CAS; (b) A. R. Hajipour, A. Zarei, L. Khazdooz and A. E. Ruoho, Synth. Commun., 2009, 39, 2702 CrossRef CAS; (c) A. Sumita, Y. Otani and T. Ohwada, Org. Biomol. Chem., 2017, 15, 9398 RSC; (d) P. E. Eaton, G. R. Carlson and J. T. Lee, J. Org. Chem., 1973, 38, 4071 CrossRef CAS; (e) D. Zewge, C.-Y. Chen, C. Deer, P. G. Domer and D. L. Hughes, J. Org. Chem., 2007, 72, 4276 CrossRef CAS PubMed.
- F. Hu, M. Patel, F. Luo, C. Flach, R. Mendelsohn, E. Garfunkel, H. He and M. Szostak, J. Am. Chem. Soc., 2015, 137, 14473 CrossRef CAS PubMed.
- M. H. Sarvari and H. Sharghi, Synthesis, 2004, 2165 Search PubMed; S. M. A. H. Siddiki, N. Rashed, A. Ali, T. Toyao, P. Hirunsit, M. Ehara and K.-i Shimizu, ChemCatChem, 2019, 11, 383 CrossRef CAS.
- M. A. Ali, S. M. A. H. Siddiki, K. Kon and K.-i Shimizu, ChemCatChem, 2015, 7, 2705 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ya00259k |
| ‡ H. B. Kim and P. K. Dutta contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |