Open Access Article
Open Access ArticleSynthesis and catalytic applications of silver nanoparticles: a sustainable chemical approach using indigenous reducing and capping agents from Hyptis capitata
R.
Revathy
a,
Jebin
Joseph
b,
Cyril
Augustine
a,
T.
Sajini
 *a and
Beena
Mathew
c
*a and
Beena
Mathew
c
aDepartment of Chemistry, St. Berchmans College (Autonomous) Campus, Mahatma Gandhi University, Kottayam, India. E-mail: sajinijose7@gmail.com
bDepartment of Botany, St. Berchmans College (Autonomous) Campus, Mahatma Gandhi University, Kottayam, India
cSchool of Chemical Sciences, Mahatma Gandhi University, Kottayam, India
First published on 21st July 2022
Abstract
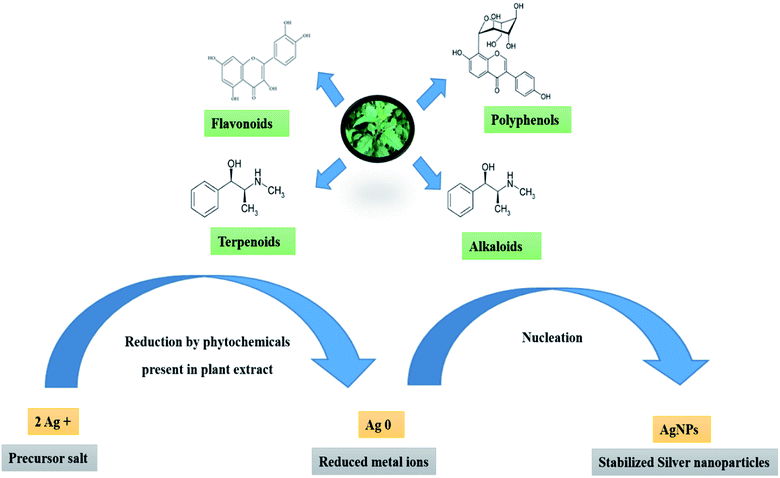
Indigenous chemical compounds found in plants such as phenolics, alkaloids, carotenoids, xanthophylls and terpenoids are used by chemists for a variety of synthetic applications. Sustainable chemistry or green chemistry, one of the recently emerged fields of science, paved the way for such chemical compounds to act as reducing and capping agents for the syntheses of numerous metal nanoparticles. In this research paper, we suggest a green protocol for the preparation of silver nanoparticles (AgNPs) using the leaf, fruit and stem extracts of Hyptis capitata, one of the commonly found plants in the tropics. The entire shoot system of this plant is used for the synthesis of AgNPs. Using microwave irradiation, AgNPs are effectively synthesized separately with leaf, fruit and stem extracts as reducing agents and the efficacy is compared. The reaction conditions such as temperature, the amount of plant extract, and the concentration of silver nitrate, one of the precursors, are optimized to establish the most efficient methodology for the synthesis. Also, the catalytic effectiveness of such nanoparticles in removing organic dyes from aqueous systems is demonstrated. Analytical methods such as UV-visible spectroscopy, HR-TEM, EDX, SAED, XRD, DLS, measurement of zeta potential, and FT-IR analysis are used to characterize the crystalline character, chemical nature and morphology of the synthesized AgNPs. The observations confirm that the entire shoot system of Hyptis capitata is a potential biomaterial for the green synthesis of AgNPs, which can be used for the removal of dyes from aqueous systems.
Environmental significanceResearch studies related to environmental issues are progressing and focusing on satisfying the updated demands of people worldwide. Nowadays people start to realize the consequences behind the conventional scientific methods of research and their influences on the ecosystem. The future of materials research undoubtedly develops with safe and environmentally benign sustainable approaches. We discuss here the synthesis of silver nanoparticles using indigenous reducing and capping agents found in Hyptis capitata. Certain catalytic applications are also presented. Instead of using chemical regents in place of reducing and capping agents, the phytochemicals are utilized. The synthetic methods applied here are eco-friendly and can be considered as an example for sustainable chemical approach or ‘green’ protocol. The AgNPs prepared from Hyptis capitata with this ecofriendly strategy are found to be an efficient nano-catalyst for the degradation of dyes. Thus the environmental pollution of several systems such as water sources due to the presence of dyes can be recognized and degraded by this methodology and it can find applications in photocatalysis, electronics, medicine, etc. |
1 Introduction
Nanotechnology is one of the outstanding fields of science, where matter is used on an atomic, molecular, and/or supramolecular scale for industrial and medical applications. Focusing on the development of nanostructures with special properties, they have wide-spread use in catalysis, microelectronics, diagnostics, bio-molecular detection, antimicrobial development, bio-sensing and the fabrication of nano-devices.1Silver based nanosystems are used in orthodontics, agriculture, the food industry and cosmetics.2,3 When antibiotics are combined with silver nanoparticles, the resultant product accomplishes remarkable capability to inhibit pathogens.4 This finding has a promising future in medical sciences since it can lead to the development of Ag-based nanoparticles incorporated with antibiotics.5,6 Existing in a restricted range of particle sizes on the nano-scale, silver-based nanoparticles possess unique qualities such as catalytic, optical, antioxidant, antimicrobial and anticancer properties.1,7–9,32–34,37
The synthesis of silver nanoparticles is achieved effectively by a variety of techniques including arc discharge, vapor deposition, pyrolysis, micro-emulsion, photo-reduction and chemical reduction of silver nitrate.4,10,11 The toxic reducing agents used in the synthetic routes and the resulting by-products are not eco-friendly and are harmful to living organisms, in most cases. The recent developments in sustainable chemistry, commonly called green chemistry, suggest the design of chemical products and processes that lessen or exclude the use and/or the generation of substances hazardous to humans, animals, plants and the environment.
The quest for green protocols for synthetic chemistry paved the way for biological methods for the synthesis of nanoparticles because of their ease, harmlessness and cost effectiveness.12 As a pre-requisite, non-toxic and renewable materials are used for implementing such protocols. Microbes including bacteria and fungi can also be utilized for the synthesis of nanoparticles.4,13,35 However, this method includes maintenance of microbial culture and purification steps. Microwave assisted methods using plant extracts as both reducing and capping agents are a sustainable approach for the rapid synthesis of metal nanoparticles.14–16,26 The scientific literature provides numerous investigations focusing on the microwave assisted synthesis of silver nanoparticles using different plant extracts.7,17 The products are reported to possess antimicrobial, anti-inflammatory and catalytic properties.38
The synthesis of nanoparticles using the components of plants is a simple, environmentally friendly, and economic approach. The adopted methodology in the present work strictly follows green protocols without using any harmful chemical reagents. The phytochemicals present in the plant extract act as both reducing and stabilizing agents. Numerous factors including the concentration of precursor, volume and concentration of the extract, reaction time, temperature, pH, plant species, and the precursor–extract ratio are found to affect the properties of the synthesized nanoparticles such as size, shape, and morphology. The technology for the large-scale synthesis of nanoparticles using plant extracts has not yet been developed fully due to the difficulties in optimizing these reaction conditions and parameters that influence the applications of synthesized nanoparticles.
Belonging to the family of Lamiaceae, Hyptis capitata, also called knobweed (Fig. 1), is one of the natural herbs found in Florida, Mexico, Central America, the West Indies, South America, Australia and Southeast Asia, and in some tropical islands.18 In many places, it is considered as a medicinal plant.19 The leaves are used to cure black diarrhoea, heart palpitations, stomach ache and many infections. Certain parts of the plant are also used for the treatment of cough, gas pain and amenorrhoea.
The leaves of Hyptis capitata are rich in a variety of phytochemicals including alkaloids, coumarins, flavonoids, glycosides, phenols, tannins, quinones, terpenoids, steroids, iridoids and saponins.18–20 These phytochemicals can be extracted successfully by using suitable polar solvents. The total concentration of phenols and flavonoids in the aqueous leaf extract of Hyptis capitata is found to be 552 mg g−1 and 0.661 mg g−1, respectively.19 Combining histochemical investigations with phytochemistry pointed out the existence of certain secretory structures in various parts of the plant.19 The catalytic, antioxidant and antimicrobial activities of Hyptis capitata arise from the unique properties of these phytochemicals. Being another widespread species of the same family, Hyptis suaveolens has been utilized for the synthesis of nanoparticles.30 The silver-based nanoparticles synthesized by means of extracts from this plant are found to possess larvicidal activities and are found to cure malaria, dengue and filariasis vectors.21,22
Silver-based nanoparticles have not yet been synthesized using the leaf, fruit and stem extracts of Hyptis capitata. As a part of this research work, extracts from Hyptis capitata are used for the synthesis of silver-based nanoparticles having the capability to function as catalysts. The indigenous compounds existing in the extract are supposed to function as capping and reducing agents during the synthetic process.
Silver nanoparticles are prepared here, using the extracts from the leaves, fruits and stem of Hyptis capitata using green protocols with microwave irradiation. The influence of change in reaction conditions such as the amount of reducing agent, concentration of the metal-ion precursor and microwave radiation is investigated thoroughly to find an optimized green-synthetic method. The synthesized nanoparticles are found to be an efficient nanocatalyst for the degradation of organic azo-dyes.
2. Materials and methods
2.1 Extraction protocols
The samples of Hyptis capitata (Fig. 1) were collected from Changanasserry, Kerala, in India. The parts of the fresh plant (Hyptis capitata) such as leaves, fruits and stems were separated and washed with copious de-ionized water. Each of the collections was sliced into small pieces and dried separately in a hot air oven. The dried samples (5 g) were placed in a round bottom flask containing 100 ml de-ionized water, and the apparatus was fitted with a condenser, and boiled for 20 minutes. The resulting extract in each case was filtered through Whatman No. 41 filter paper. These extracts obtained from leaves, fruits and stems (Fig. 2) were used for further experiments discussed below. | ||
| Fig. 2 Phytochemicals extracted to aqueous media from the (a) leaves, (b) fruits, and (c) stem of Hyptis capitata. | ||
2.2 Synthesis of silver nanoparticles
The three extracts obtained above from the leaves, fruits and stem of Hyptis capitata were used for the reduction of Ag(I) ions to Ag(0).For this, 10 ml of the extract from the leaves was added to 90 ml of aqueous silver nitrate (1 mM) solution and the resulting mixture was subjected to microwave irradiation for 3 minutes in a domestic microwave oven [Sharp R219T (W)] operating at 800 W power and 2450 MHz frequency. The formation of AgNPs was monitored using a UV-visible spectrophotometer keeping an interval of 30 seconds. Following the above methodology, AgNPs were synthesized also from the fruits and stem extracts (Scheme 1).
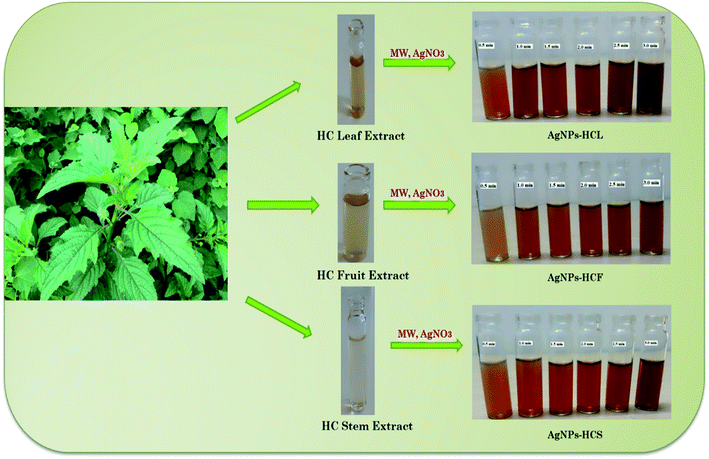 | ||
| Scheme 1 Green synthesis of AgNPs from Hyptis capitata (HC) from (a) leaf, (b) fruit, and (c) stem extracts. | ||
2.3 Strategies adopted for the optimization of reaction conditions
Aiming at the effective synthesis of AgNPs with maximum yield, the influence of three major factors was analyzed experimentally, which include the role of microwave irradiation, the amount of the plant extract, and the concentration of silver nitrate.To assess the importance of microwave irradiation, each reaction mixture was kept at room temperature for 3 hours. Small aliquots were withdrawn at regular intervals of 30 minutes and analyzed by UV-visible spectroscopy.
Similarly, the amount of plant extract is also varied and the formation of AgNPs is traced. This is done by using 20 and 30 ml of extracts in each case (leaves, fruits and stem) for the preparation of the reaction mixture.
Likewise, the influence of the concentration of silver nitrate was studied by repeating the experiments using solutions of 2–5 mM silver nitrate with 10 ml of the extracts.
2.4 Characterization of silver nanoparticles
The surface plasmon resonance of the synthesized silver nanoparticles has been analyzed by using a UV-visible spectrophotometer. The colloid consisting of silver nanoparticles was diluted with distilled water and loaded into a quartz cuvette. The intensity of various electronic transitions was traced between 200 and 600 nm. The whole study was performed with a Shimadzu UV-2450 spectrophotometer.The FT-IR spectra of the synthesized silver nanoparticles were recorded using a Perkin Elmer-400 spectrometer with an ATR attachment. The characteristic vibrational frequencies of the various chemical bonds existing in different functional groups present in the initial plant extracts and the resultant nanoparticles synthesized from leaf, fruit and stem extracts were obtained in a range of 500–3500 cm−1.31
TEM is used to determine the particle size distribution and average size of particles on the nanometer scale of the synthesized nanoparticles. For TEM analysis, a JEOL JEM-2100 microscope with an EDX attachment was used. Images of samples were recorded at different magnifications.
The hydrodynamic size of the synthesized nanoparticles was observed with a Dynamic Light Scattering Detector (DLS).23 The surface charge and stability were also examined.
For the better understanding of the crystal planes, X-ray diffraction studies of AgNPs were carried out. Applying a scan rate of 1.2° per minute, the XRD studies were conducted on a PANalytical XPERT-PRO X-ray spectrometer and the angles were measured from 20° to 78°. A nickel filter and CuK (1.5418 A0) radiation were used in the experiments. The stability of the synthesized zero-valent silver nanoparticles in aqueous solution was determined by zeta potential.24,25
2.5 Catalytic activity of silver nanoparticles
The catalytic efficiency of AgNPs-HC (Hyptis capitata) was examined by observing the reduction of methyl orange with sodium borohydride in the presence of the synthesized AgNPs. Solutions of 0.5 ml of 0.06 M NaBH4, 2 ml of 0.1 × 10 −3 M methyl orange and 0.5 ml AgNPs-HC were mixed in a quartz cell. Using UV-visible spectrophotometry, the change in the concentration of methyl orange as a function of time was monitored between 200 and 600 nm at 30 minute intervals.3. Results and discussion
Simple, cost-effective and eco-friendly methods increase the interest in the synthesis of metal nanoparticles, which find emerging applications worldwide. The implemented methodology in this research paper follows green protocols without any harmful chemical reagents. The major observations related to this work are presented below with adequate discussion.3.1 Synthesis of silver nanoparticles
Silver nanoparticles were initially synthesized by irradiating a mixture of 90 ml of 1 mM AgNO3 solution and 10 ml HC plant extract with microwave irradiation. No color change was observed while mixing the plant extract and AgNO3 solution. The color of the reaction mixture gradually changed from colorless to yellowish brown indicating the formation of AgNPs16 (Fig. 3).3.2 Electronic spectra of the synthesized AgNPs-HC
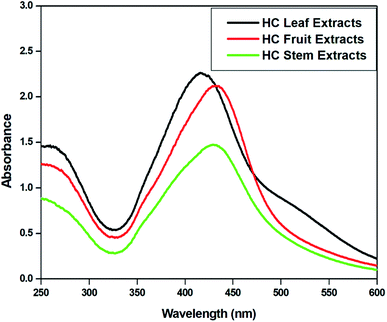
The UV-vis absorption spectra of the reaction mixtures were recorded in the range 200–800 nm at an interval of 30 seconds and are depicted in Fig. 4. All the spectra exhibit an absorption band in the range of 400–450 nm, which is the typical surface plasmon resonance (SPR) band of AgNPs due to the coupling between the electron cloud on the surface of AgNPs with the applied electromagnetic radiation. The absorbance of AgNPs-HCL is relatively high in comparison with the others confirming the formation of more AgNPs from the HC leaf extract.3.3 Effect of microwave irradiation on the synthesis of AgNPs
The effect of microwave irradiation on the rate of formation of nanoparticles was examined in detail. The AgNPs were synthesized from plant extracts at room temperature without microwave irradiation. The reaction mixture was kept at room temperature for 3 hours and UV-visible spectra were taken at an interval of 30 minutes [Fig. 5(a–c)]. For AgNPs-HCL, the intensity of the absorption maximum gradually increased and the formation of AgNPs was found to be completed after 3 hours. The absorbance values revealed that the concentration of AgNPs synthesized at room temperature was relatively less in comparison with the amount of AgNPs obtained by microwave irradiation. All three systems revealed the formation of AgNPs at room temperature even though AgNPs-HCL showed higher absorbance values compared to the others.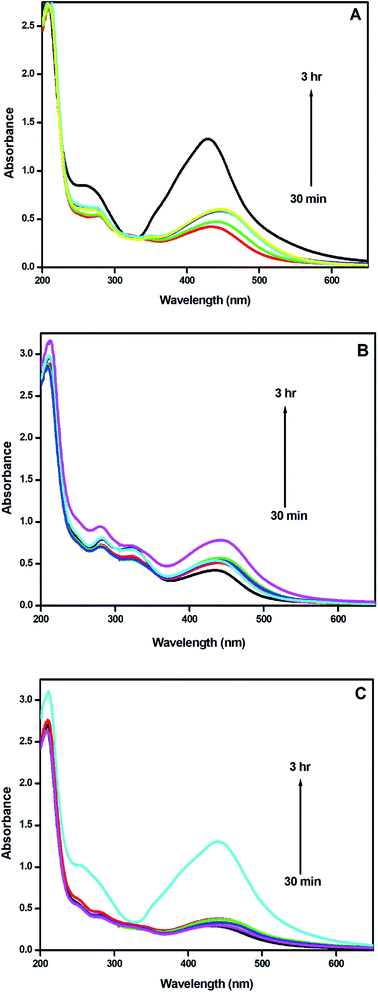 | ||
| Fig. 5 UV-visible spectra of AgNPs-HC synthesized at room temperature: (a) AgNPs-HCL, (b) AgNPs-HCF, and (c) AgNPs-HCS. | ||
At room temperature, the absorption maximum observed at 436 nm shifted slightly towards longer wavelengths as a function of time, confirming the increase in the size of the nanoparticles formed. The microwave assisted method yields stable and smaller nanoparticles more rapidly in comparison with those obtained by the synthesis done at room temperature. The results convey that the former method is more suitable for the green synthesis of silver nanoparticles from Hyptis capitata plant extracts.
3.4 Effect of the concentration of plant extracts
The effect of the concentration of the selected plant extract was examined by making nanoparticles with 10, 20 and 30 ml of the extract and 90 ml of silver nitrate aqueous solution using microwave irradiation and the corresponding electronic spectra are recorded (Fig. 6). It was found that the number of nanoparticles obtained is increased and consequently the absorbance values are enhanced. As the concentration of the extract increases the absorbance of the transition at 250 nm in the extract is also increased. The phytochemicals present in the extracts enhance the reduction of silver ions and thus more AgNPs are formed in the colloid with high stability. The flavonoids, alkaloids and polyphenols present in the Hyptis capitata plant extract are responsible for the stability of colloidal dispersions of the AgNPs synthesized.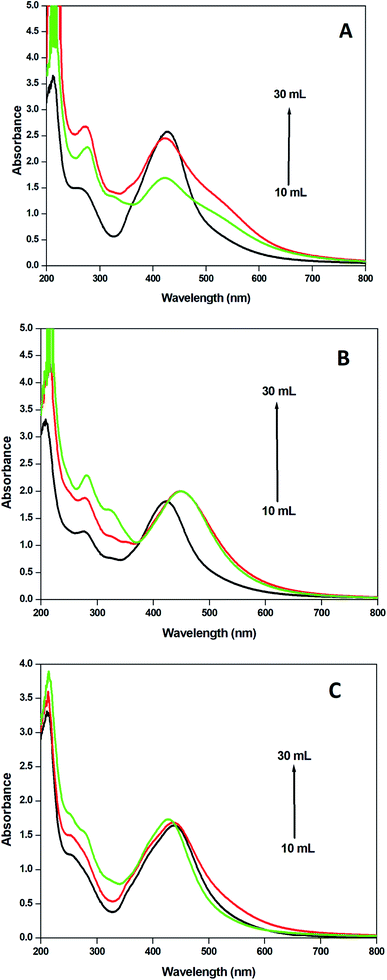 | ||
| Fig. 6 UV-visible spectra of AgNPs recorded from solutions obtained by mixing 90 ml (1 mM) of AgNO3 solution with 10–30 ml of extracts from (a) leaves, (b) fruits, and (c) stems. | ||
3.5 Effect of the concentration of AgNO3
The concentration of the precursor is an important factor in the synthesis of nanoparticles. The formation of nanoparticles commences, when the concentration of the precursor in the medium is reached an optimum range for nucleation. The importance of the concentration of silver nitrate was studied by preparing nanoparticles with 1–5 mM aqueous solution of silver nitrate and 10 ml of plant extracts from each category. The result obtained revealed the formation of more nanoparticles with the increased silver nitrate concentration. It was supported by the increase in the absorption of the SPR band in the UV-visible spectra (Fig. 7).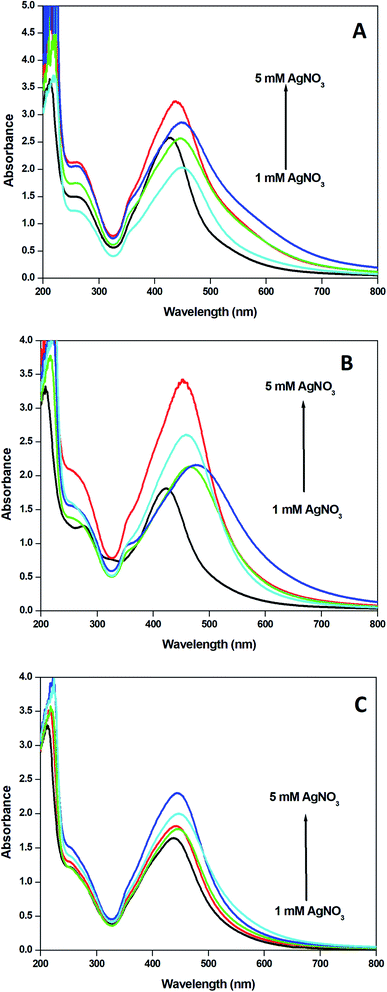 | ||
| Fig. 7 UV-visible spectra of AgNPs from solutions obtained by mixing 1–5 mM AgNO3 solution and 10 ml of (a) leaf extract, (b) fruit extract and (c) stem extract. | ||
3.6 Characterization of AgNPs
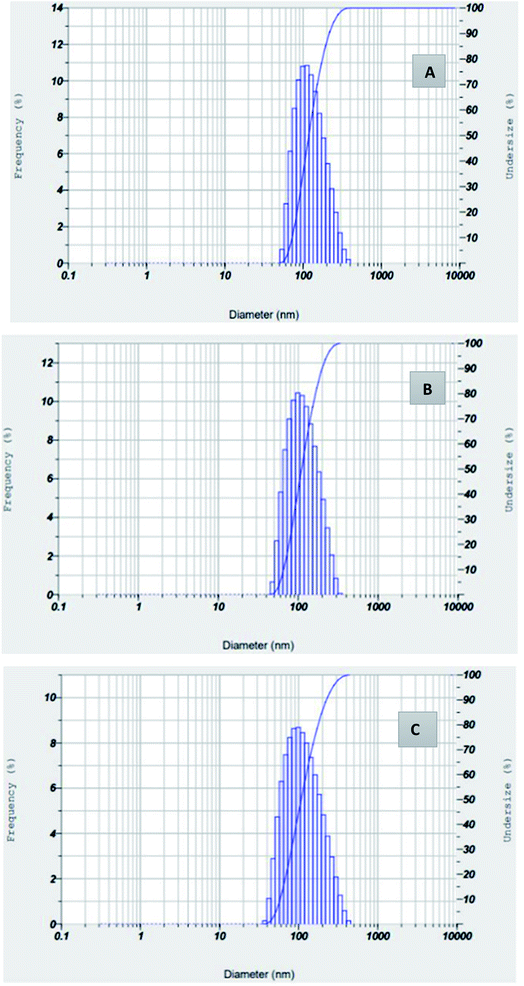
The hydrodynamic size of silver nanoparticles synthesized from Hyptis capitata leaf, fruit and stem extracts was determined using DLS analysis (Fig. 8).23 The average particle size of AgNPs-HCF, AgNPs-HCL, and AgNPs-HCS is given in Table 1. All these results indicated monodisperse nanoparticle formation. The value of Z-average is more for AgNPs-HCF, which is supported by results obtained in other spectroscopic analyses.| Property | AgNPs-HCF | AgNPs-HCL | AgNPs-HCS |
|---|---|---|---|
| Z-average (nm) | 167.3 | 104.4 | 94.4 |
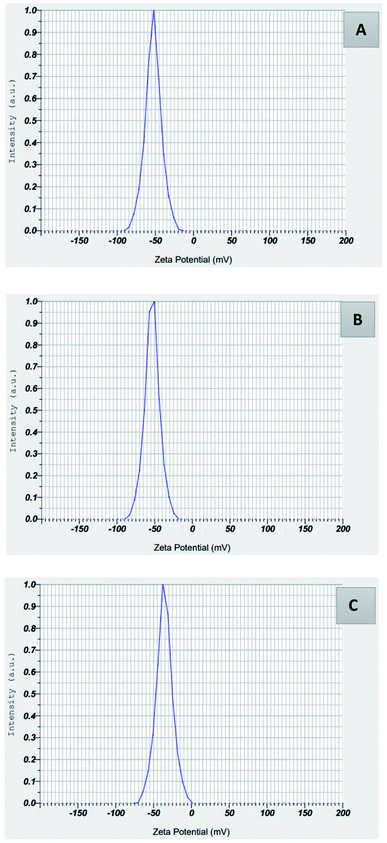
The stability of the synthesized zero-valent silver nanoparticles in aqueous solution was determined by zeta potential.24 In a liquid suspension, the zeta potential conveys the electrostatic attraction or repulsion between particles. Zeta potential values for AgNPs-HCF, AgNPs-HCL, and AgNPs-HCS (Fig. 9) are given in Table 2.
| Property | AgNPs-HCF | AgNPs-HCL | AgNPs-HCS |
|---|---|---|---|
| Mean value of zeta potential (mV) | −52.8 | −53.3 | −35.8 |
The synthesized nanoparticles are capped with the hydroxyl group of polyphenols, which makes the zeta potential values negative. In the absence of any steric stabilization, particles with zeta potential lower than −30 mV can give long term stability for a colloidal dispersion. The strong anions, negatively charged hydroxyl groups, are on the surface of nanoparticles, and give stability by capping, which is also confirmed by the FT-IR spectroscopic analysis. Also, negatively charged particles are relatively nontoxic to the environment.23
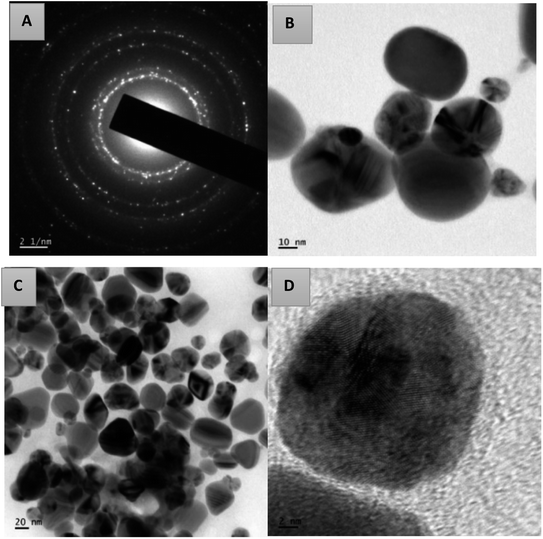
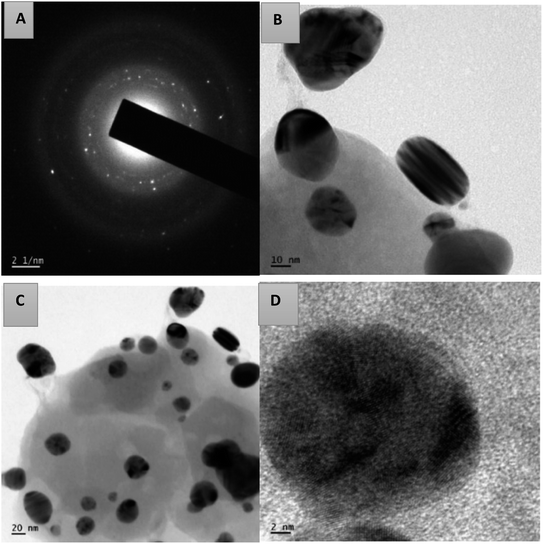
Using transmission electron microscopy, the size and shape of the synthesized silver nanoparticles were examined. The TEM images of AgNPs-HCF, AgNPs-HCL and AgNPs-HCS are depicted in Fig. 10–12, respectively. The nanoparticles are spherical and oval in shape. On the (111) plane, silver nanoparticles have grown preferentially, which can be seen from the lattice fringes in the image.27 The selected area electron diffraction (SAED) pattern has circular rings, which indicates silver nanoparticles in a face centred cubic structure.28 The (111) reflection ring is more intense and closer to the center. The (200), (220) and (311) reflections are clearly visible. The synthesized silver nanoparticles are polycrystalline, which is confirmed by the clear circular rings obtained in the SAED pattern.
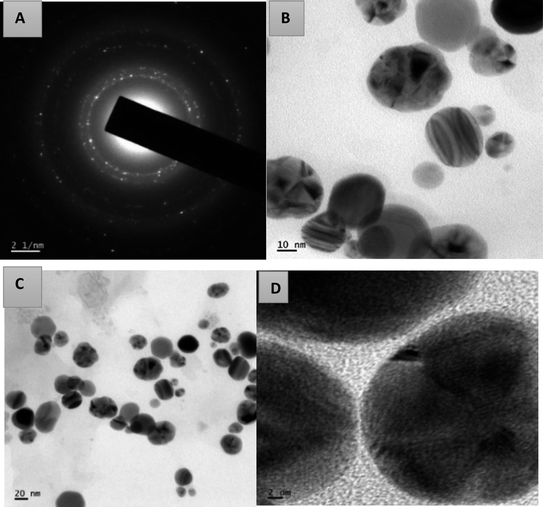 | ||
| Fig. 10 The SAED pattern of AgNPs-HCF (a) and HR-TEM images with different magnifications of AgNPs-HCF (b–d). | ||
 | ||
| Fig. 11 The SAED pattern of AgNPs-HCL (a) and HR-TEM images with different magnifications of AgNPs-HCL (b–d). | ||
 | ||
| Fig. 12 The SAED pattern of AgNPs-HCS (a) and HR-TEM images with different magnifications of AgNPs-HCS (b–d). | ||
From the HR-TEM images, it is clear that the AgNPs formed are not uniformly distributed in the colloid.
The circular rings are perfectly aligned in the SAED pattern of AgNPs-HCL compared to the pattern obtained for the other cases, which indicates poly-crystallinity.
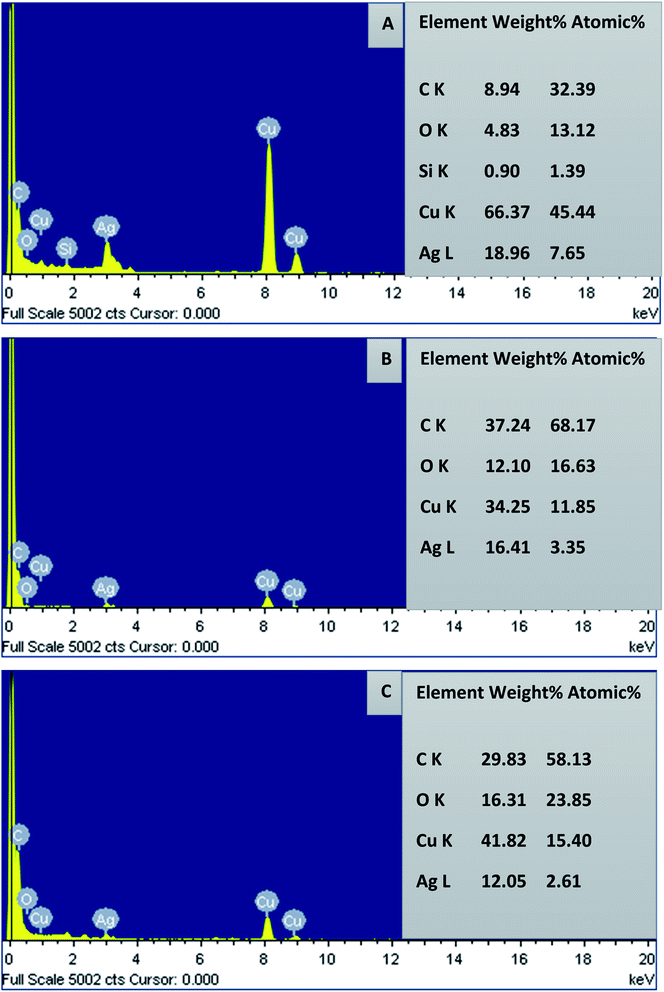
By subjecting the TEM grid to energy dispersive X-ray spectroscopy (EDX) of HR-TEM, the elemental constituents of AgNPs were measured and are presented in Fig. 13. The strong absorption peak located at 3 keV proves the presence of elemental silver in the nanoparticles. The AgNPs were successfully capped by the extracellular constituents of Hyptis capitata extracts on the surface of nanoparticles, which was confirmed by the peaks obtained for carbon and oxygen. The signals for carbon and copper also originate from the carbon coated copper grid used in TEM sample preparation and EDX analysis. Compared to AgNPs-HCF and AgNPs-HCS, elemental silver is more present in AgNPs-HCL. The peak obtained for copper is more intense for AgNPs-HCL and an exclusive peak for silicon is also found. This is due to the presence of these elements in the leaf extract only. Relatively weak signals for copper are found in the spectrum of AgNPs-HCF and AgNPs-HCS.
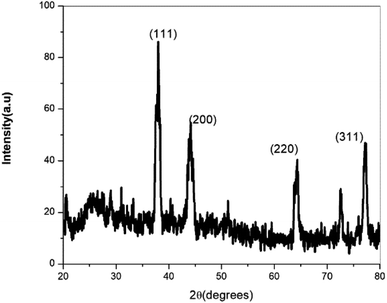
The crystalline nature of AgNPs was confirmed by X-ray diffraction (Fig. 14). The XRD image shows four peaks at 2θ values of 38.36, 44.52, 64.66, and 77.66. Comparing this with ICDD 04-0783, the above peaks can be assigned to the (111), (200), (220), and (311) reflections of face centred cubic silver nanoparticles. Using the Scherrer formula, the average particle size was calculated to be 15 nm.29 The data from X-ray diffraction confirm that the silver nanoparticles formed in this method are crystalline in nature.
The FT-IR spectra of Hyptis capitata leaf, fruit and stem extracts and the respective AgNPs are depicted in Fig. 15.
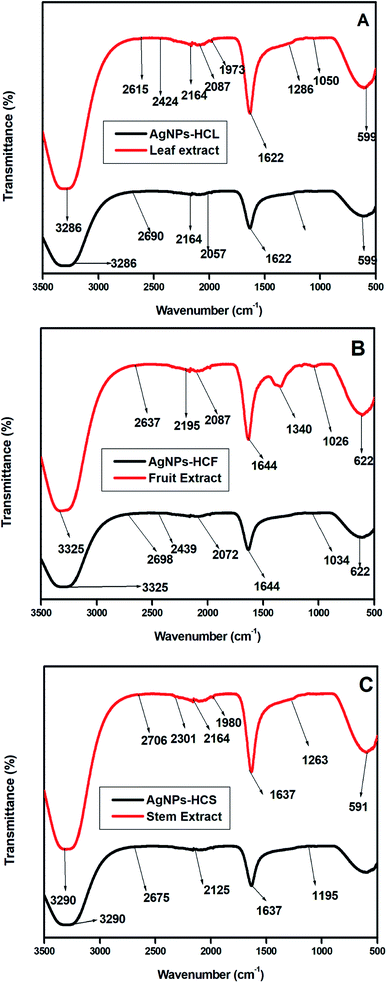 | ||
| Fig. 15 FT-IR spectra of (a) the HC leaf extract and AgNPs-HCL, (b) the HC fruit extract and AgNPs-HCF, and (c) the HC stem extract and AgNPs-HCS. | ||
The association of plant extracts with the nanoparticles is examined by using FT-IR spectra. Similar peaks are obtained for leaf, fruit and stem extracts. An additional intense peak at 1361 cm−1 is obtained for the fruit extract, which is due to the –C–O stretching vibrations from carboxyl groups. A broad band obtained in the 3400–3200 cm−1 range is due to the stretching vibrations of the hydroxyl group of various metabolites such as flavonoids and polyphenols present in Hyptis capitata plant extracts. The absorption peak at 1627 cm−1 represents the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of aromatic rings. The characteristic C–O stretching vibrations of alcohols appear as a strong absorption band at 1036 cm−1 in the fruit extract. The peaks obtained at 606 cm−1, 622 cm−1 and 584 cm−1 indicate the presence of halo-alkanes.
C stretching vibrations of aromatic rings. The characteristic C–O stretching vibrations of alcohols appear as a strong absorption band at 1036 cm−1 in the fruit extract. The peaks obtained at 606 cm−1, 622 cm−1 and 584 cm−1 indicate the presence of halo-alkanes.
Characteristic hydrogen bonded alcoholic stretching vibrations at 3286 cm−1 are obtained in the FT-IR spectrum of the synthesized silver nanoparticles. The peak at 1630 cm−1 is due to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the conjugated ring. The FT-IR spectra of AgNPs synthesized from all three extracts show similar peaks.
C in the conjugated ring. The FT-IR spectra of AgNPs synthesized from all three extracts show similar peaks.
The FT-IR spectrum of synthesized AgNPs-HC shows a slight decrease in the wavenumber of these peaks. This indicates the chemical reaction between silver nitrate and the plant extract. Different functional groups present in the plant extract are responsible for the reduction of silver nitrate and capping of the surface of silver nanoparticles.36 This is the reason for the stability of AgNPs in the colloid.
3.7 Catalytic activity
The catalytic activity of metal nanoparticles is usually studied by observing the reduction of organic dyes by NaBH4. A dye has different colors in the oxidized and reduced forms. The SPR band of metal nanoparticles should not be interfered with by the absorption maximum of dye. For the catalytic study of silver nanoparticles, methyl orange is selected here. An aqueous solution of methyl orange is orange red in color; after reduction it is colorless. An absorption maximum at 464 nm due to the presence of the azo-group is observed for methyl orange in the UV-Visible spectrum.8 The SPR of silver nanoparticles is separated from this. In the absence of the silver nanocatalyst, the reduction of methyl orange by NaBH4 is too slow such that we can see that the peak intensity remains unchanged for several hours.17 Immediately after the addition of the catalyst, the color of methyl orange faded. A change in the peak intensity was observed. In the presence of the nanocatalyst, the reaction rate is enhanced. The reduction of methyl orange catalyzed by silver nanoparticles was monitored in an interval of 30 minutes with UV-visible spectroscopy and is presented in Fig. 16. All the spectra showed similar degradation patterns. Among the different extracts, AgNPs-HCL exhibited high catalytic activity towards the reduction of methyl orange.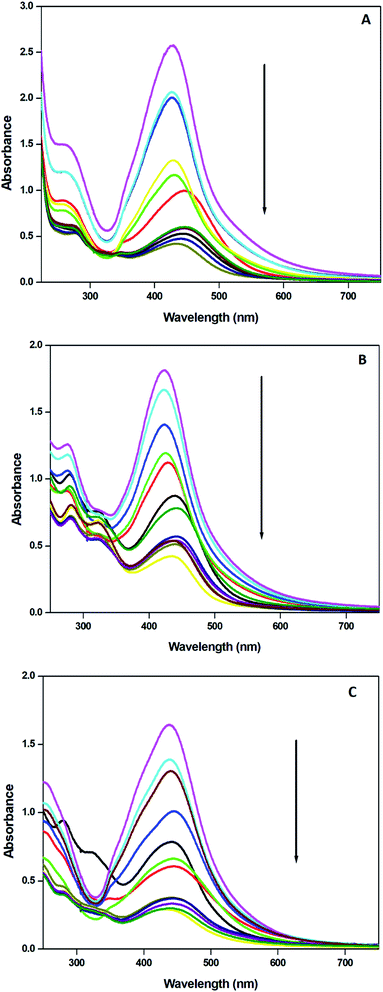 | ||
| Fig. 16 UV-visible spectra indicating the catalytic reduction of methyl orange by sodium borohydride in the presence of (a) AgNPs-HCL, (b) AgNPs-HCF and (c) AgNPs-HCS. | ||
Gradually the absorbance was found to be decreased in the presence of the nanocatalyst. This confirms the decrease in the concentration of methyl orange. The reaction was completed in 3 hours, which shows that the presence of silver nanoparticles catalyzed the reduction of the azo-dye. Otherwise, it takes several hours to get a change in the UV-visible absorption spectrum of methyl orange.
A potential mechanism for the formation of AgNPs from HC extracts is depicted in Fig. 17. The –OH and –COOH groups present in the polyhydroxy compounds in the plant extract may be responsible for the bio-reduction of silver ions. The oxidized components may be absorbed on the surface of AgNPs through the electrostatic force of attraction between –COOH groups and hydrogen bonds with the neighbouring molecules.
In summary, the silver nanoparticles can be rapidly synthesized by irradiating a mixture of HC shoot extracts and silver nitrate solution with microwave irradiation. At room temperature, the process is found to be time consuming. The concentration of each component of the mixture influences the number of nanoparticles formed. The AgNPs synthesized are extensively characterized by several spectroscopic methods. The entire shoot system of the HC plant is effective for the preparation of AgNPs in an eco-friendly method. AgNPs-HCL absorbs more in the UV visible spectrum. The other characterization methods also reveal that it contains more zero valent silver atoms. Thus, the catalytic efficiency of AgNPs-HCL is relatively high in comparison with the other extracts.
4 Conclusions
The present study demonstrated a rapid green approach to the synthesis of AgNPs using Hyptis capitata plant extracts, which supplies a cost-effective, simple and eco-friendly method for the synthesis of AgNPs. Here the entire shoot system of Hyptis capitata was utilized for the formation of AgNPs. Metabolites such as phenols and flavonoids present in plant extracts were mainly responsible for the biosynthesis of AgNPs. The presence of elemental silver in the nanoparticles was confirmed by the absorption peak obtained at 3 keV in the EDX spectra of AgNPs. The UV-visible spectrum of AgNPs-HCL showed the maximum absorbance compared to AgNPs-HCF and AgNPs-HCS, which indicates the high concentration of AgNPs formed. The absorbance values in the UV-visible spectra obtained for these nanoparticles were found to be relatively less at room temperature after 3 hours. Microwave assisted synthesis is a rapid method to prepare smaller AgNPs. With the increasing amount of plant extract, we get more AgNPs, indicating that the phytochemicals in them enhance the reduction of silver ions. More AgNPs were formed with the increase in the concentration of silver nitrate in the solution. This method yielded face centered cubic, spherical and oval AgNPs with high stability. The AgNPs so prepared exhibited good catalytic efficiency, suggesting that AgNPs might be useful as an agent for dye removal from aqueous systems. AgNPs-HCL is found to be a more efficient nanocatalyst, since the absorbance of methyl orange is reduced more in the corresponding UV-visible spectra.Data availability statement
Data are available upon reasonable request.Funding
This research received no external funding.Conflicts of interest
There are no conflicts to declare.References
- K. M. M. Abou El-Nour, A. Eftaiha, A. Al-Warthan and R. A. A. Ammar, Synthesis and applications of silver nanoparticles, Arabian J. Chem., 2010, 3, 135–140, DOI:10.1016/j.arabjc.2010.04.008.
- S. H. Lee and B. H. Jun, Silver nanoparticles: Synthesis and application for nanomedicine, Int. J. Mol. Sci., 2019, 20, 865, DOI:10.3390/ijms20040865.
- A. C. Burduşel, O. Gherasim, A. M. Grumezescu, L. Mogoantă, A. Ficai and E. Andronescu, Biomedical applications of silver nanoparticles: An up-to-date overview, Nanomaterials, 2018, 8, 1–25, DOI:10.3390/nano8090681.
- M. Murphy, K. Ting, X. Zhang, C. Soo and Z. Zheng, Current Development of Silver Nanoparticle Preparation, Investigation, and Application in the Field of Medicine, J. Nanomater., 2015, 2015 DOI:10.1155/2015/696918.
- B. Khodashenas and H. R. Ghorbani, Synthesis of silver nanoparticles with different shapes, Arabian J. Chem., 2019, 12, 1823–1838, DOI:10.1016/j.arabjc.2014.12.014.
- K. S. Siddiqi, A. Husen and R. A. K. Rao, A review on biosynthesis of silver nanoparticles and their biocidal properties, J. Nanobiotechnol., 2018, 16, 14, DOI:10.1186/s12951-018-0334-5.
- K. Seku, B. R. Gangapuram, B. Pejjai, K. K. Kadimpati and N. Golla, Microwave-assisted synthesis of silver nanoparticles and their application in catalytic, antibacterial and antioxidant activities, J. Nanostruct. Chem., 2018, 8, 179–188, DOI:10.1007/s40097-018-0264-7.
- S. Joseph and B. Mathew, Microwave Assisted Biosynthesis of Silver Nanoparticles Using the Rhizome Extract of Alpinia galanga and Evaluation of Their Catalytic and Antimicrobial Activities, J. Nanopart., 2014, 2014, 1–9, DOI:10.1155/2014/967802.
- Y. He, X. Li, Y. Zheng, Z. Wang, Z. Ma, Q. Yang, B. Yao, Y. Zhao and H. Zhang, A green approach for synthesizing silver nanoparticles, and their antibacterial and cytotoxic activities, New J. Chem., 2018, 42, 2882–2888, 10.1039/c7nj04224h.
- M. I. Masum, M. M. Siddiqa, K. A. Ali, Y. Zhang, Y. Abdallah, E. Ibrahim, W. Qiu, C. Yan and B. Li, Biogenic synthesis of silver nanoparticles using phyllanthus emblicafruit extract and its inhibitory action against the pathogen acidovorax oryzaestrain RS-2 of rice bacterial brown stripe, Front. Microbiol., 2019, 10, 1–18, DOI:10.3389/fmicb.2019.00820.
- M. Yusuf, Silver Nanoparticles: Synthesis and Applications, ( 2019) 2343–2356 Search PubMed.
- S. Wei, Y. Wang, Z. Tang, J. Hu, R. Su, J. Lin, T. Zhou, H. Guo, N. Wang and R. Xu, A size-controlled green synthesis of silver nanoparticles by using the berry extract ofSea Buckthorn and their biological activities, New J. Chem., 2020, 44, 9304–9312, 10.1039/d0nj01335h.
- F. Okafor, A. Janen, T. Kukhtareva, V. Edwards and M. Curley, Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity, Int. J. Environ. Res. Public Health, 2013, 10, 5221–5238, DOI:10.3390/ijerph10105221.
- S. Saha, M. M. Malik and M. S. Qureshi, Microwave Synthesis of Silver Nanoparticles, Nano Hybrids, 2013, 4, 99–112, DOI:10.4028/www.scientific.net/nh.4.99.
- S. Joseph and B. Mathew, Microwave assisted facile green synthesis of silver and gold nanocatalysts using the leaf extract of Aerva lanata, Spectrochim. Acta, Part A, 2015, 136, 1371–1379, DOI:10.1016/j.saa.2014.10.023.
- D. Singh, D. Rawat and Isha, Microwave-assisted synthesis of silver nanoparticles from Origanum majorana and Citrus sinensis leaf and their antibacterial activity: a green chemistry approach, Bioresour. Bioprocess., 2016, 3, 14, DOI:10.1186/s40643-016-0090-z.
- S. Joseph and B. Mathew, Microwave-assisted facile synthesis of silver nanoparticles in aqueous medium and investigation of their catalytic and antibacterial activities, J. Mol. Liq., 2014, 197, 346–352, DOI:10.1016/j.molliq.2014.06.008.
- V. Sumitha, K. Murugan and I. Mini, Physico-Chemical and Phytochemical Evaluation of a Medicinal Herb Hyptis capitata Jacq. (Lamiaceae), BioSci. Trends, 2018, 11, 1188–1193 Search PubMed.
- Y. Sulistyaningsih and D. Ratnadewi, Identification of Secretory Structure, Histochemstry and Phytochemical Compounds of medicinal Plant Hyptis capitata Jacq, Biotropia, 2017, 24, 94–103, DOI:10.11598/btb.201.
- I. W. Kusuma, Rahmini, E. T. Arung, A. Y. Pramono and S. Erwin, Biological activities and phytochemicals of hyptis capitata grown in east kalimantan, indonesia, J. Appl. Biol. Biotechnol., 2020, 8, 58–64, DOI:10.7324/JABB.2020.80210.
- T. Fafal, P. Taştan, B. S. Tüzün, M. Ozyazici and B. Kivcak, Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Asphodelus aestivus Brot. aerial part extract, South African J. Bot., 2017, 112, 346–353, DOI:10.1016/j.sajb.2017.06.019.
- H. Ashraf, T. Anjum, S. Riaz and S. Naseem, Microwave-Assisted Green Synthesis and Characterization of Silver Nanoparticles Using Melia azedarach for the Management of Fusarium Wilt in Tomato, Front. Microbiol., 2020, 11, 1–22, DOI:10.3389/fmicb.2020.00238.
- K. Jyoti, M. Baunthiyal and A. Singh, Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics, J. Radiat. Res. Appl. Sci., 2016, 9, 217–227, DOI:10.1016/j.jrras.2015.10.002.
- S. Ahmed, U. Saif, M. Ahmad, B. L. Swami and S. Ikram, Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract, J. Radiat. Res. Appl. Sci., 2016, 9, 1–7, DOI:10.1016/j.jrras.2015.06.006.
- M. A. Xavier, D. P. Gelain, F. A. Silva, J. S. Quintans, M. F. Agra and A. A. S. Arau, Synthesis of Silver Nanoparticles by Chemical Reduction Method: Effect of Reducing Agent and Surfactant Concentration, Int. J. Automot. Mech. Eng., 2011, 14, 1389–1396 Search PubMed.
- K. J. Sreeram, M. Nidhin and B. U. Nair, Microwave assisted template synthesis of silver nanoparticles, Bull. Mater. Sci., 2008, 31, 937–942, DOI:10.1007/s12034-008-0149-3.
- Hemlata, P. R. Meena, A. P. Singh and K. K. Tejavath, Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity against Cancer Cell Lines, ACS Omega, 2020, 5, 5520–5528, DOI:10.1021/acsomega.0c00155.
- B. Satyanarayana and D. P. Subhashini, Green synthesis of silver nanoparticles using Hyptis suaveolens (L.) Poit leaf extracts, their characterization and cytotoxicity evaluation against PC-3 and MDA-MB 231 cells, Biologia, 2019, 74, 783–793, DOI:10.2478/s11756-019-00222-1.
- V. Benjamín, E. B. Salasa and V. M. PartidaaSilver nanoparticles from Hpytus suaveolens and their effect on the biochemical and physiological parameters in mesquite plants, Biocatalysis and Agricultural Biotechnology ISSN: 1878-8181, 2020, vol. 28, 11, p. 101733 Search PubMed.
- D. Elumalai, M. Hemavathi, C. V. Deepaa and P. K. Kaleena, Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors, Parasite Epidemiol Control, 2017, 2(4), 15–26, DOI:10.1016/j.parepi.2017.09.001.
- V. Selvaraj, S. Sagadevan and L. Muthukrishnan, Eco-friendly approach in the synthesis of silver nanoparticles and evaluation of optical, surface morphological and antimicrobial properties, J. Nanostruct. Chem., 2019, 9, 153–162, DOI:10.1007/s40097-019-0306-9.
- S. Sudip, S. Biraj, B. Kinkar and K. J. Tushar, Bio-molecule functionalized rapid one-pot green synthesis of silver nanoparticles and their efficacy toward the multidrug-resistant (MDR) gut bacteria of silkworms (Bombyx mori), RSC Adv., 2020, 10, 22742–22757 RSC.
- S. Some, O. Bulut, K. Biswas, A. Kumar and A. Roy, Effect of feed supplementation with biosynthesized silver nanoparticles using leaf extract of Morus indica L. V1 on Bombyx mori L. (Lepidoptera: Bombycidae), Mater. Res. Express, 2019, 6, 012001 CrossRef.
- D. A. Berrak, S. K. Gokce and O. Ismail, Extracellular directed Ag NPs formation and investigation of their antimicrobial and cytotoxic properties, Saudi Pharm. J., 2019, 27(Issue 1), 9–16 Search PubMed.
- S. A. Mohd, P. Jitendra and Y. Yeoung-Sang, Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts, ACS Sustainable Chem. Eng., 2013, 1(6), 591–602 CrossRef.
- S. U. Ilay, D. Ayse, O. Irem, I. Nilay and O. Ismail, One-step preparation of stable gold nanoparticle using red cabbage extracts under UV light and its catalytic activity, J. Photochem. Photobiol., B, 2020, 204, 111800, DOI:10.1016/j.jphotobiol.2020.111800.
- C. Ramazan, D. Ayse, O. Ismail and A. Abdurrahman, Green synthesis of silver nanoparticles using aqueous extracts of three Sideritis species from Turkey and evaluations bioactivity potentials, Sustainable Chem. Pharm., 2021, 21, 100426, DOI:10.1016/j.scp.2021.100426.
- O. Ismail, L. P. Mathews, A. O. Muserref, K. Sanju, C. Tao, Y. Mingxu and T. Weihong, Nanotechnology in Plant Disease Management: DNA-Directed Silver Nanoparticles on Graphene Oxide as an Antibacterial against Xanthomonas perforans, ACS Nano, 2013, 7(10), 8972–8980, DOI:10.1021/nn4034794.
| This journal is © The Royal Society of Chemistry 2022 |