Balancing the performance and stability of organic photodiodes with all-polymer active layers†
Abstract
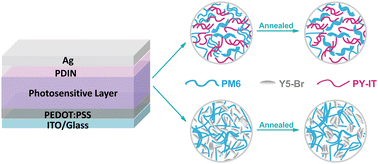
Organic photodiodes (OPDs) have emerged as a potential alternative for inorganic photodiodes in light-detection technology due to their unique properties such as lightweight, flexibility, and tunable absorption range. However, the stability of OPDs is one of the main issues preventing the commercialization of OPDs. Herein, we performed a detailed characterization study of OPDs composed of all-polymer and polymer donors/small molecular acceptors to evaluate the detectivity and stability of devices. The small molecule acceptor Y5-Br and its polymerized counterpart PY-IT were selected to minimize the energy and absorption differences and paired with PM6. We found that the Y5-Br based device exhibited a higher detectivity and a lower dark current density due to the lesser extent of the trap density, whereas the PY-IT based device demonstrated a faster response time and impressive thermal/electrical stability. In addition, the analysis of the photosensitive layer morphology reveals that the all-polymer films showed remarkable thermal tolerance. In contrast, the thermal treatment resulted in apparent polymer and small molecule aggregations in the polymer–small molecule counterpart, leading to large scale phase segregations. Our findings suggest that the all-polymer composition is an efficient strategy to improve the stability of OPDs and provide a useful guidance for the commercial application of OPDs in the future.
- This article is part of the themed collection: Stability of Optoelectronic Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...