Significant alternation of molecular structures and properties in quinoidal conjugated polymers by chalcogen atom substitution†
Abstract
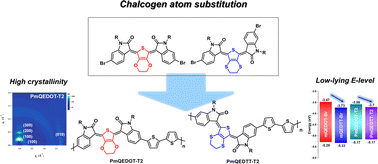
Quinoid derivatives can be applied to various applications as organic semiconducting materials owing to their unique intrinsic optoelectronic properties compared to those of existing aromatic compounds. Structural modification of the quinoid core would effectively control the optoelectronic properties and suppress the inevitable geometrical isomerism. In this study, two quinoidal building blocks mQEDTT-Br and mQEDOT-Br, incorporating 3,4-ethylenedithiothiophene (EDTT) or 3,4-ethylenedioxythiophene (EDOT), respectively, were synthesized for systematic investigation of chalcogen atom substitution effect in quinoid cores. The substitution of O with S atoms in the quinoid core extended the π-conjugation pathway and introduced a π-electron accepting capability in the quinoid. The S-based quinoid exhibited red-shifted light absorption and lower frontier molecular orbital (FMO) energy levels with a narrowed band gap (∼0.2 eV). Instead, O-containing quinoid exhibited a single isomer form and higher quinoid character, which is favorable for intermolecular packing, leading to effective charge transport. Two novel quinoidal conjugated polymers, PmQEDTT-T2 and PmQEDOT-T2, were successfully synthesized. Grazing incidence wide-angle X-ray scattering (GIWAXS) results indicated that the PmQEDOT-T2 film showed highly ordered crystallinity compared to the PmQEDTT-T2 film, confirming that mQEDOT has a higher quinoid character than that of mQEDTT, which is beneficial for charge transport. Organic field-effect transistors (OFETs) using PmQEDOT-T2 demonstrated a higher hole mobility of 0.13 cm2 V−1 s−1 than that of PmQEDTT-T2 (0.02 cm2 V−1 s−1). Therefore, atomic substitution on the quinoid core is a simple structural modification that can control the presence and type of isomers, quinoid characters, and consequent device performance as desired.
- This article is part of the themed collection: Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...