Enhancing the luminescence performance of Ca2−xSrxBO3Cl:Eu2+,Dy3+ by substitution of Sr2+ for Ca2+†
Abstract
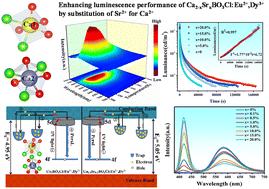
As reusable materials, persistent luminescence (PersL) materials have a wide range of applications in emergency response, information encryption and other fields. However, there is still a lack of commercial PersL materials with a yellow afterglow in current studies. Based on this, a series of Ca2−xSrxBO3Cl:Eu2+,Dy3+ samples were synthesized by a high temperature solid-state method, and then their crystal structure, optical performance and trap properties are discussed in this work. First, EPR and XPS showed that Sr2+ substitution is beneficial for the generation of oxygen vacancy traps, which would improve the luminescence performance of the samples from 12 h to 46 h. Next, through PL and XANES it was found that the characteristic emission of oxygen vacancies and Eu2+ emission presented color-adjustable PL properties, and also produced single matrix white light, which solved the stroboscopic problem of the blue chip in LEDs. In addition, the emission of oxygen vacancies can transfer energy to Eu2+ excitation, which marks the first report on the energy transfer of oxygen vacancies. Finally, the ML properties of the samples were investigated, providing a new method for enhancing the luminescence properties of samples.
- This article is part of the themed collection: Stability of Optoelectronic Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...