Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A theoretical study of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/h2_char_0033_0304.gif) m phases of Sb2S3, Bi2S3, and Sb2Se3
m phases of Sb2S3, Bi2S3, and Sb2Se3
E. Lora
da Silva
 *a,
J. M.
Skelton
*a,
J. M.
Skelton
 b,
P.
Rodríguez-Hernández
b,
P.
Rodríguez-Hernández
 c,
A.
Muñoz
c,
A.
Muñoz
 c,
M. C.
Santos
de,
D.
Martínez-García
f,
R.
Vilaplana
c,
M. C.
Santos
de,
D.
Martínez-García
f,
R.
Vilaplana
 g and
F. J.
Manjón
g and
F. J.
Manjón
 e
e
aIFIMUP, Departamento de Física e Astronomia, Faculdade de Ciênicas da Universidade do Porto, 4169-007, Porto, Portugal. E-mail: estelina.silva@fc.up.pt; Fax: +351 22 04 02 406; Tel: +351 22 04 02 362
bDepartment of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK
cDepartamento de Física, Instituto de Materiales y Nanotecnología, MALTA Consolider Team, Universidad de La Laguna, 38206, Tenerife, Spain
dSede do Agrupamento Escolas de Ponte de Sor, 7400-259, Ponte de Sor, Portugal
eInstituto de Diseño para la Fabricación y Producción Automatizada, MALTA Consolider Team, Universitat Politècnica de València, 46022, València, Spain
fDepartamento de Física Aplicada – ICMUV, MALTA Consolider Team, Universitat de València, 46100, Burjassot, Spain
gCentro de Tecnologías Físicas, MALTA Consolider Team, Universitat Politècnica de València, 46022, València, Spain
First published on 22nd September 2022
Abstract
We report a comparative theoretical study of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2S3, Bi2S3, and Sb2Se3 close to ambient pressure. Our enthalpy calculations at 0 K show that at ambient pressure the R
m phases of Sb2S3, Bi2S3, and Sb2Se3 close to ambient pressure. Our enthalpy calculations at 0 K show that at ambient pressure the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (tetradymite-like) phase of Sb2Se3 is energetically more stable than the Pnma phase, contrary to what is observed for Sb2S3 and Bi2S3, and irrespective of the exchange–correlation functional employed in the calculations. The result for Sb2Se3 is in contradiction to experiments in which all three compounds are usually grown in the Pnma phase. This result is further confirmed by free-energy calculations taking into account the temperature dependence of unit-cell volumes and phonon frequencies. Lattice dynamics and elastic tensor calculations further show that both the Pnma and R
m (tetradymite-like) phase of Sb2Se3 is energetically more stable than the Pnma phase, contrary to what is observed for Sb2S3 and Bi2S3, and irrespective of the exchange–correlation functional employed in the calculations. The result for Sb2Se3 is in contradiction to experiments in which all three compounds are usually grown in the Pnma phase. This result is further confirmed by free-energy calculations taking into account the temperature dependence of unit-cell volumes and phonon frequencies. Lattice dynamics and elastic tensor calculations further show that both the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3 are dynamically and mechanically stable at zero applied pressure. Since these results suggest that the formation of the R
m phases of Sb2Se3 are dynamically and mechanically stable at zero applied pressure. Since these results suggest that the formation of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 should be feasible under close to ambient conditions, we provide a theoretical crystal structure and simulated Raman and infrared spectra to help in its identification. We also discuss the results of the two published works that have claimed to have synthesized tetradymite-like Sb2Se3. Finally, the stability of the R
m phase of Sb2Se3 should be feasible under close to ambient conditions, we provide a theoretical crystal structure and simulated Raman and infrared spectra to help in its identification. We also discuss the results of the two published works that have claimed to have synthesized tetradymite-like Sb2Se3. Finally, the stability of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase across the three group-15 A2X3 sesquichalcogenides is analysed based on their van der Waals gap and X–X in-plane geometry.
m phase across the three group-15 A2X3 sesquichalcogenides is analysed based on their van der Waals gap and X–X in-plane geometry.
1 Introduction
Since the identification of the trigonal tetradymite-like R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of group-15 sesquichalcogenides (i.e. Sb2Te3, Bi2Se3, and Bi2Te3; Fig. 1a) as 3D topological insulators,1,2 the family of A2X3 sesquichalcogenides has attracted a great deal of attention from the scientific community. Three-dimensional topological insulators represent a new class of matter, with insulating bulk electronic states and topologically protected metallic surface states arising from time-reversal symmetry and strong spin–orbit coupling (SoC). These properties make them of potential interest for spintronics and quantum computing applications.3 Due to this fundamental interest and potential applications, the identification of new topological insulators and materials with superconducting properties is currently an important research area in condensed matter science.
m phases of group-15 sesquichalcogenides (i.e. Sb2Te3, Bi2Se3, and Bi2Te3; Fig. 1a) as 3D topological insulators,1,2 the family of A2X3 sesquichalcogenides has attracted a great deal of attention from the scientific community. Three-dimensional topological insulators represent a new class of matter, with insulating bulk electronic states and topologically protected metallic surface states arising from time-reversal symmetry and strong spin–orbit coupling (SoC). These properties make them of potential interest for spintronics and quantum computing applications.3 Due to this fundamental interest and potential applications, the identification of new topological insulators and materials with superconducting properties is currently an important research area in condensed matter science.
The stibnite (Sb2S3), bismuthinite (Bi2S3), and antimonselite (Sb2Se3) minerals are also group-15 sesquichalcogenides but do not crystallize in the tetradymite-like R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure under ambient conditions and instead are reported to adopt an orthorhombic U2S3-type Pnma structure (Fig. 1b). Sb2S3, Bi2S3, and Sb2Se3 are semiconductors with band gap widths of 1.7, 1.3, and 1.2 eV, respectively.4,5 These materials are used in a wide range of technological applications including photovoltaics (solar cells), X-ray computed tomography detectors, fuel cells, gas sensors, and for the detection of biomolecules.6–12 Additionally, Sb2Se3 has recently found a number of other applications including in solid-state batteries, fiber lasers, and photoelectrochemical devices.13–16
m structure under ambient conditions and instead are reported to adopt an orthorhombic U2S3-type Pnma structure (Fig. 1b). Sb2S3, Bi2S3, and Sb2Se3 are semiconductors with band gap widths of 1.7, 1.3, and 1.2 eV, respectively.4,5 These materials are used in a wide range of technological applications including photovoltaics (solar cells), X-ray computed tomography detectors, fuel cells, gas sensors, and for the detection of biomolecules.6–12 Additionally, Sb2Se3 has recently found a number of other applications including in solid-state batteries, fiber lasers, and photoelectrochemical devices.13–16
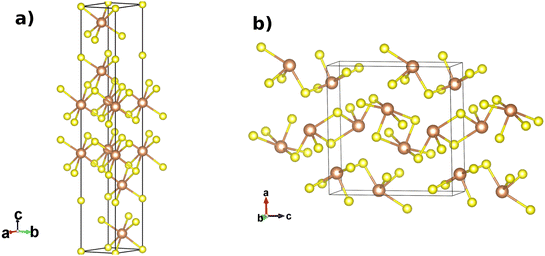 | ||
Fig. 1 Structures of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (a) and Pnma (b) phases of the A2X3 sesquichalcogenides (A = Sb, Bi and X = S, Se). The A cations and X anions are shown as brown and yellow spheres, respectively. m (a) and Pnma (b) phases of the A2X3 sesquichalcogenides (A = Sb, Bi and X = S, Se). The A cations and X anions are shown as brown and yellow spheres, respectively. | ||
Since several phases, including the Pnma phase, have been synthesized for Bi2Se3, which usually crystallizes in the tetradymite-like R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure,17–20 it is natural to wonder whether the R
m structure,17–20 it is natural to wonder whether the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure could be adopted by other sesquichalcogenides that generally adopt the U2S3-type structure, viz. Sb2S3, Bi2S3, and in particular Sb2Se3. In fact, several theoretical studies have been performed over the years to investigate the properties of the hypothetical tetradymite-like Sb2Se3 structure. Some of these studies have suggested that this phase should undergo a topological quantum phase transition under compression,21,22 while one found that tetradymite-like Sb2Se3 is dynamically stable and is a topological insulator at ambient pressure.23 Interestingly enough, the R
m structure could be adopted by other sesquichalcogenides that generally adopt the U2S3-type structure, viz. Sb2S3, Bi2S3, and in particular Sb2Se3. In fact, several theoretical studies have been performed over the years to investigate the properties of the hypothetical tetradymite-like Sb2Se3 structure. Some of these studies have suggested that this phase should undergo a topological quantum phase transition under compression,21,22 while one found that tetradymite-like Sb2Se3 is dynamically stable and is a topological insulator at ambient pressure.23 Interestingly enough, the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of group-15 sesquichalcogenides are characterized by a unique type of bonding termed “metavalent bonding” that not only underpins the topological properties but also makes these materials useful for phase-change memories, as highly efficient thermoelectrics, and for photonic devices.24
m phases of group-15 sesquichalcogenides are characterized by a unique type of bonding termed “metavalent bonding” that not only underpins the topological properties but also makes these materials useful for phase-change memories, as highly efficient thermoelectrics, and for photonic devices.24
In 2013 an experimental study claimed to have observed the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3, with a topological transition occurring at 2 GPa,25 but the tetradymite-like structure of Sb2Se3 was not confirmed beyond doubt. On the other hand, a comparative experimental and theoretical study of the three U2S3-type sesquichalcogenides suggested the Pnma structure to be stable up to 50 GPa.26 This is supported by several experimental high-pressure studies on Sb2Se3 in which the Pnma structure was found to be stable up to 50 GPa and above.27,28 However, one study observed a pressure-induced isostructural phase transition at 12 GPa and a further transition to a disordered Im
m phase of Sb2Se3, with a topological transition occurring at 2 GPa,25 but the tetradymite-like structure of Sb2Se3 was not confirmed beyond doubt. On the other hand, a comparative experimental and theoretical study of the three U2S3-type sesquichalcogenides suggested the Pnma structure to be stable up to 50 GPa.26 This is supported by several experimental high-pressure studies on Sb2Se3 in which the Pnma structure was found to be stable up to 50 GPa and above.27,28 However, one study observed a pressure-induced isostructural phase transition at 12 GPa and a further transition to a disordered Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure above 50 GPa, followed by a pressure-induced amorphization on releasing the pressure.29 Despite the apparent stability of the Pnma phase, experimental high-pressure studies have also found that Pnma Sb2Se3 becomes a topological superconductor at 2.5 K and around 10 GPa,30 exhibiting highly conducting spin-polarized surface states similar to those observed for Bi2Se3.31 Furthermore, a recent study has claimed to have synthesized R
m structure above 50 GPa, followed by a pressure-induced amorphization on releasing the pressure.29 Despite the apparent stability of the Pnma phase, experimental high-pressure studies have also found that Pnma Sb2Se3 becomes a topological superconductor at 2.5 K and around 10 GPa,30 exhibiting highly conducting spin-polarized surface states similar to those observed for Bi2Se3.31 Furthermore, a recent study has claimed to have synthesized R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m of Sb2Se3 by atomic layer epitaxy on a buffer layer of Bi2Se3.32 We can therefore conclude that while the bulk of the experimental evidence suggests that the R
m of Sb2Se3 by atomic layer epitaxy on a buffer layer of Bi2Se3.32 We can therefore conclude that while the bulk of the experimental evidence suggests that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 is not observed at high pressures, it is not conclusive as to whether this phase could potentially be formed under favourable synthesis conditions.
m phase of Sb2Se3 is not observed at high pressures, it is not conclusive as to whether this phase could potentially be formed under favourable synthesis conditions.
In light of the above studies, it is interesting to compare the stabilities of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structural phases of the three U2S3-type sesquichalcogenides under close to ambient conditions and to confirm whether or not the R
m structural phases of the three U2S3-type sesquichalcogenides under close to ambient conditions and to confirm whether or not the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase could be synthetically accessible. In this work, we report a set of systematic density-functional theory (DFT) calculations on the Pnma and R
m phase could be synthetically accessible. In this work, we report a set of systematic density-functional theory (DFT) calculations on the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of the three U2S3-type sesquichalcogenides under ambient conditions and at pressures up to 10 GPa. We show that the Pnma phases of Sb2S3 and Bi2S3 are energetically more stable than the R
m phases of the three U2S3-type sesquichalcogenides under ambient conditions and at pressures up to 10 GPa. We show that the Pnma phases of Sb2S3 and Bi2S3 are energetically more stable than the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases over this pressure range, but that, unexpectedly, the R
m phases over this pressure range, but that, unexpectedly, the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 is predicted by several exchange–correlation (XC) functionals to be more stable than the Pnma phase close to ambient conditions. To aid in future experimental efforts to prepare the R
m phase of Sb2Se3 is predicted by several exchange–correlation (XC) functionals to be more stable than the Pnma phase close to ambient conditions. To aid in future experimental efforts to prepare the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3, we also confirm its dynamical and mechanical stability and provide a theoretical structure and vibrational spectra to support its identification. Finally, we also discuss the only two studies that, to our knowledge, have claimed to have prepared the tetradymite-like phase of Sb2Se3 under close to ambient conditions.
m phase of Sb2Se3, we also confirm its dynamical and mechanical stability and provide a theoretical structure and vibrational spectra to support its identification. Finally, we also discuss the only two studies that, to our knowledge, have claimed to have prepared the tetradymite-like phase of Sb2Se3 under close to ambient conditions.
2 Methods
The structural properties of the different crystalline phases of Sb2S3, Bi2S3, and Sb2Se3 were calculated within the framework of pseudopotential plane-wave density-functional theory33 using the Vienna Ab initio Simulation Package (VASP) code.34 The revised Perdew–Burke–Ernzerhof generalized-gradient approximation (GGA) functional for solids (PBEsol)35,36 was used for all calculations. Additional calculations were also performed with the local-density approximation (LDA)37 functional and the dispersion-corrected PBE-D238 functional to assess the impact of the XC treatment on the results. Projector augmented-wave (PAW) pseudopotentials including six valence electrons for S[3s2 3p4] and Se[4s2 4p4] and fifteen valence electrons for Sb[4d10 5s2 5p3] and Bi[5d10 6s2 6p3] were used to model the ion cores. The convergence of the total energy was achieved with a plane-wave kinetic-energy cut-off of 600 eV. The Brillouin zones (BZs) were sampled with Γ-centered Monkhorst-Pack39 grids with 6 × 10 × 6 (Pnma) and 12 × 12 × 12 subdivisions (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).
m).
Structural relaxations were performed by allowing the atomic positions and the unit-cell parameters to optimise at a series of fixed volumes in order to confirm the stability of both the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases over a pressure range from 0–10 GPa. At each volume, we obtain the (hydrostatic) external pressure for the applied compression and the corresponding structural parameters. The pressure–volume (p–V) curves for each of the compounds were fitted to a third-order Birch–Murnaghan equation of state40,41 to obtain the equilibrium volume, the bulk modulus, and its pressure derivative. The enthalpy H as a function of volume was computed using the relationship H = E + pV, where E is the total electronic energy of the system, p is the pressure, and V is the volume. Comparison of the H curves of the different polymorphs can provide insight into the relative thermodynamic stabilities over the studied pressure range.
m phases over a pressure range from 0–10 GPa. At each volume, we obtain the (hydrostatic) external pressure for the applied compression and the corresponding structural parameters. The pressure–volume (p–V) curves for each of the compounds were fitted to a third-order Birch–Murnaghan equation of state40,41 to obtain the equilibrium volume, the bulk modulus, and its pressure derivative. The enthalpy H as a function of volume was computed using the relationship H = E + pV, where E is the total electronic energy of the system, p is the pressure, and V is the volume. Comparison of the H curves of the different polymorphs can provide insight into the relative thermodynamic stabilities over the studied pressure range.
Lattice-dynamics calculations were performed on the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3 at a series of cell volumes corresponding to different applied pressures. The phonon frequencies were computed by using the supercell finite-displacement method implemented in the Phonopy package42 with VASP as the force calculator.43 Supercell expansions of 2 × 4 × 2 for the Pnma phase and 2 × 2 × 2 for the R
m phases of Sb2Se3 at a series of cell volumes corresponding to different applied pressures. The phonon frequencies were computed by using the supercell finite-displacement method implemented in the Phonopy package42 with VASP as the force calculator.43 Supercell expansions of 2 × 4 × 2 for the Pnma phase and 2 × 2 × 2 for the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases were used to enable the exact calculation of frequencies at the zone center (Γ) and unique zone-boundary wave vectors, which were interpolated to obtain phonon-dispersion curves together with density of states curves on uniform 50 × 50 × 50 Γ-centered q-point meshes.
m phases were used to enable the exact calculation of frequencies at the zone center (Γ) and unique zone-boundary wave vectors, which were interpolated to obtain phonon-dispersion curves together with density of states curves on uniform 50 × 50 × 50 Γ-centered q-point meshes.
Infrared (IR) and Raman spectra were calculated for the ground-state R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 using the methods described in ref. 44 and implemented in the Phonopy-Spectroscopy package.45 The spectral linewidths were obtained by computing the third-order force constants of a 2 × 2 × 2 expansion of the primitive cell, and following the many-body perturbative approach described in detail in ref. 46 and implemented in the Phono3py software.
m phase of Sb2Se3 using the methods described in ref. 44 and implemented in the Phonopy-Spectroscopy package.45 The spectral linewidths were obtained by computing the third-order force constants of a 2 × 2 × 2 expansion of the primitive cell, and following the many-body perturbative approach described in detail in ref. 46 and implemented in the Phono3py software.
Elastic tensors were computed to assess the mechanical stability of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3 at zero pressure, by employing the central-difference method where the unique components of the elastic tensor are determined by performing six finite distortions of the lattice and deriving the tensor elements from the strain–stress relationship.47 For these calculations, it was necessary to increase the plane-wave energy cutoff to 950 eV to converge the stress tensor. We then employed the ELATE software48 to analyze the linear compressibility using the results.
m phases of Sb2Se3 at zero pressure, by employing the central-difference method where the unique components of the elastic tensor are determined by performing six finite distortions of the lattice and deriving the tensor elements from the strain–stress relationship.47 For these calculations, it was necessary to increase the plane-wave energy cutoff to 950 eV to converge the stress tensor. We then employed the ELATE software48 to analyze the linear compressibility using the results.
3 Results and discussion
3.1 Structural properties of the Pnma phase
In order to verify the accuracy of our calculations as a prior step before attempting to study the potential R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2S3, Bi2S3 and Sb2Se3, we first calculated the equilibrium lattice parameters, bulk moduli and pressure derivatives of the Pnma phases and compared them to other experimental and theoretical studies in the literature (Table 1).
m phases of Sb2S3, Bi2S3 and Sb2Se3, we first calculated the equilibrium lattice parameters, bulk moduli and pressure derivatives of the Pnma phases and compared them to other experimental and theoretical studies in the literature (Table 1).
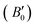 of the Pnma phases of Sb2Se3, Sb2S3 and Bi2S3 compared to experiments and other theoretical results from the literature
of the Pnma phases of Sb2Se3, Sb2S3 and Bi2S3 compared to experiments and other theoretical results from the literature
| Sb2Se3 | Sb2S3 | Bi2S3 | ||||
|---|---|---|---|---|---|---|
| Theo. | Exp. | Theo. | Exp. | Theo. | Exp. | |
| a This work. b Ref. 26. c Ref. 49. d Ref. 50. e Ref. 51. f Ref. 27. g Ref. 52. h Ref. 53. i Ref. 54. j Ref. 55. k Ref. 56. l Ref. 57. m Ref. 58. n Ref. 59. o Ref. 60. p Ref. 61. q Ref. 62. r Ref. 63. s Ref. 30. t Ref. 64. u Ref. 65. | ||||||
| a 0 (Å) | 11.75a | 11.24a | 11.19a | |||
| 11.80b | 11.80f | 11.27b | 11.30bjk | 11.41b | 11.27p | |
| 11.52c | 11.79g | 11.02c | 11.31lm | 11.00n | 11.33q | |
| 11.91d | 11.30h | 11.58o | ||||
| 11.53e | 11.08i | |||||
| b 0 (Å) | 3.98a | 3.83a | 3.96a | |||
| 3.99b | 3.98f | 3.81c | 3.84bjk | 3.97b | 3.97p | |
| 3.96ce | 3.99g | 3.84h | 3.84lm | 3.94n | 3.98q | |
| 3.98d | 3.83bi | 3.99o | ||||
| c 0 (Å) | 11.30a | 10.91a | 10.94a | |||
| 11.28b | 11.65fg | 10.89b | 11.23bjlm | 11.01b | 11.13p | |
| 11.22ce | 10.79c | 11.24k | 10.83n | 11.18q | ||
| 11.70d | 11.22h | 11.05o | ||||
| 10.81i | ||||||
| V 0 (Å3) | 528.11a | 469.6a | 484.4a | |||
| 531.1b | 547.1f | 470.4b | 486.0b | 498.3b | 498.4p | |
| 511.8c | 547.5g | 453.0c | 487.7imj | 469.1n | 501.6q | |
| 598.1r | 552.5s | 529.9r | 488.2k | 510.1o | ||
| 511.6q | ||||||
| B 0 (GPa) | 31.1a | 31.5a | 42.3a | |||
| 70.5c | 30.0f | 32.2b | 37.6b | 83.6n | 36.6p | |
| 32.7s | 80.3c | 26.9j | 32.3o | 38.9q | ||
| 27.2k | 36.5q | 37.5u | ||||
| 41.4t | ||||||
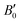
|
6.6a | 6.6a | 6.8a | |||
| 6.1f | 6.2b | 3.8b | 5.9q | 6.4p | ||
| 5.6s | 7.9j | 6.4o | 5.5q | |||
| 6.0k | 4.6u | |||||
| 7.8t | ||||||
The Pnma phases of A2X3 sesquichalcogenides comprise layers stacked by weak interactions along the crystallographic a direction, the description of which is challenging for conventional DFT functionals.26,51,63
The calculated lattice parameters of Sb2Se3 (a0 = 11.75 Å, b0 = 3.98 Å and c0 = 11.30 Å) are in good agreement with the experimental measurements in ref. 27 and 52 (a0 = 11.80 Å, b0 = 3.97 Å, c0 = 11.65 Å and a0 = 11.79 Å, b0 = 3.98 Å and c0 = 11.65 Å, respectively), and also with other ab initio calculations.26,49–51 The most notable deviation of our calculated values from experimental measurements is a ∼3% reduction of the c0 parameter, which contributes to a ∼3–4% underestimation of V0 compared to experiments. Our results are comparable to the theoretical results in ref. 26, where calculations were also carried out using PAW pseudopotentials and the PBEsol functional. The c0 of 11.70 Å quoted in ref. 50 is considerably larger than the present results but closer to experiments, but the a0 parameter has a larger error. We attribute this to the use of the PBE functional in this study, which has a tendency to overestimate volumes and has been shown to do so by ∼10% for antimony chalcogenides.63 On the other hand, the LDA tends to underestimate volumes, as can be seen in the lattice parameters quoted in ref. 49, 51 and 59. Interestingly, the difference in the predicted and measured b-axis lengths is very small, which we attribute to the fact that this crystallographic direction corresponds to covalently bonded chains of atoms.
Our calculated lattice parameters for the Pnma phase of Sb2S3 show similar trends to those of Sb2Se3. As shown in Table 1, the calculated parameters agree well with experimental measurements26,55 and other theoretical results.26,49,53,54 We note that, however, for Sb2Se3, the lattice parameters obtained from LDA calculations tend to underestimate compared to experiments, resulting in discrepancies with the a0 quoted in ref. 49 and 54, although the c0 parameter is closer to our PBEsol results than to the PBE values quoted in ref. 53, which again agree better with experimental results.26,55
Our results for Bi2S3 are also consistent with experimental measurements and other theoretical studies in the literature. We note that calculations performed on Bi2S3 using the Armiento and Mattsson 2005 parametrized GGA functional (AM05)66–68 seem to show a slightly better reproduction of the c0 parameter compared to experiments.26
The calculated B0 and 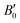 values obtained by fitting the p–V curves of Sb2Se3 to a third-order Birch–Murnaghan equation are B0 = 31.1 GPa (
values obtained by fitting the p–V curves of Sb2Se3 to a third-order Birch–Murnaghan equation are B0 = 31.1 GPa (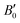 = 6.6), which are close to the experimental values of B0 = 30 GPa (
= 6.6), which are close to the experimental values of B0 = 30 GPa (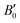 = 6.1) from ref. 27 and B0 = 32.7 GPa (
= 6.1) from ref. 27 and B0 = 32.7 GPa (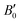 = 5.6) from ref. 60. For Sb2S3, we obtained B0 = 31.5 GPa (
= 5.6) from ref. 60. For Sb2S3, we obtained B0 = 31.5 GPa (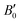 = 6.6), which is within the range of experimental values26,55,56,64 and consistent with the PAW/PBEsol calculations in ref. 26 These results are also close to those experimentally measured for the As-doped stibnite mineral.69 Finally, our values of B0 = 42.3 GPa (
= 6.6), which is within the range of experimental values26,55,56,64 and consistent with the PAW/PBEsol calculations in ref. 26 These results are also close to those experimentally measured for the As-doped stibnite mineral.69 Finally, our values of B0 = 42.3 GPa (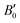 = 6.8) for Bi2S3 are again consistent with other DFT calculations60,62 and the experimental values reported in ref. 61, 62 and 65.
= 6.8) for Bi2S3 are again consistent with other DFT calculations60,62 and the experimental values reported in ref. 61, 62 and 65.
3.2 Energetic stability of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m phases at 0 K and up to 10 GPa
m phases at 0 K and up to 10 GPa
Since our calculations on the Pnma phases were found to be in good agreement with experimental and theoretical studies, we proceeded to carry out a theoretical study of the potential R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2S3, Bi2S3, and Sb2Se3 to probe whether this phase could be energetically competitive from ambient pressure up to 10 GPa.
m phases of Sb2S3, Bi2S3, and Sb2Se3 to probe whether this phase could be energetically competitive from ambient pressure up to 10 GPa.
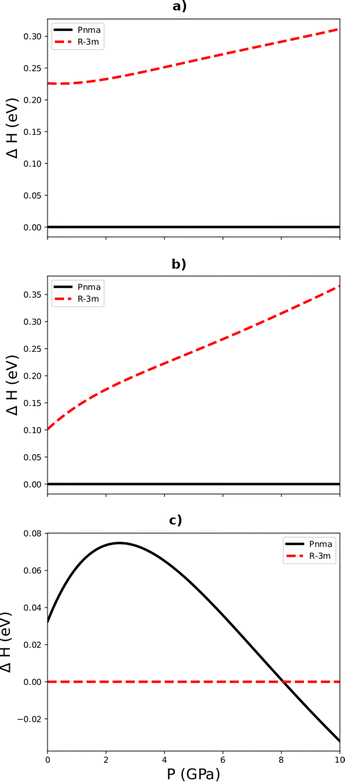
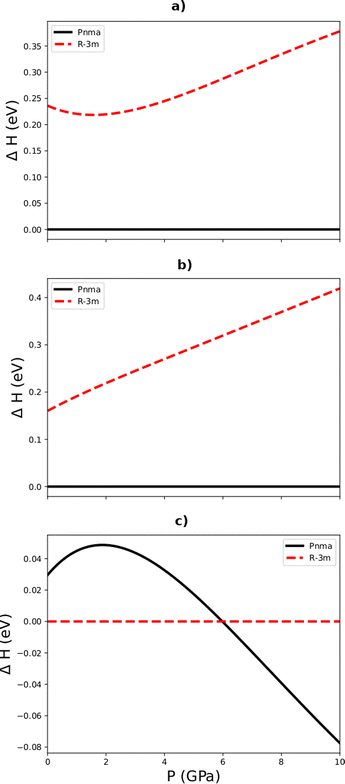
Fig. 2a–c show the pressure dependence of the enthalpy differences between the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and Pnma phases of Sb2S3, Bi2S3, and Sb2Se3, respectively. We found that the orthorhombic Pnma phase is the most energetically stable phase of Bi2S3 and Sb2S3 at all the pressures examined, as expected from experiments that obtained this phase both under ambient conditions and at high pressures. Surprisingly, however, our simulations predict that the R
m and Pnma phases of Sb2S3, Bi2S3, and Sb2Se3, respectively. We found that the orthorhombic Pnma phase is the most energetically stable phase of Bi2S3 and Sb2S3 at all the pressures examined, as expected from experiments that obtained this phase both under ambient conditions and at high pressures. Surprisingly, however, our simulations predict that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 is more stable than the Pnma phase below 4.8 GPa, indicating that both the Pnma and R
m phase of Sb2Se3 is more stable than the Pnma phase below 4.8 GPa, indicating that both the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases are energetically competitive over this range. This in principle contradicts existing experimental studies on Sb2Se3 that have so far consistently obtained the Pnma phase under ambient conditions. We note, however, that the energy difference between the two phases is only 22.71 meV per f.u., which is lower than kBT ≈ 25 meV at 300 K and which should therefore make it accessible under ambient conditions.
m phases are energetically competitive over this range. This in principle contradicts existing experimental studies on Sb2Se3 that have so far consistently obtained the Pnma phase under ambient conditions. We note, however, that the energy difference between the two phases is only 22.71 meV per f.u., which is lower than kBT ≈ 25 meV at 300 K and which should therefore make it accessible under ambient conditions.
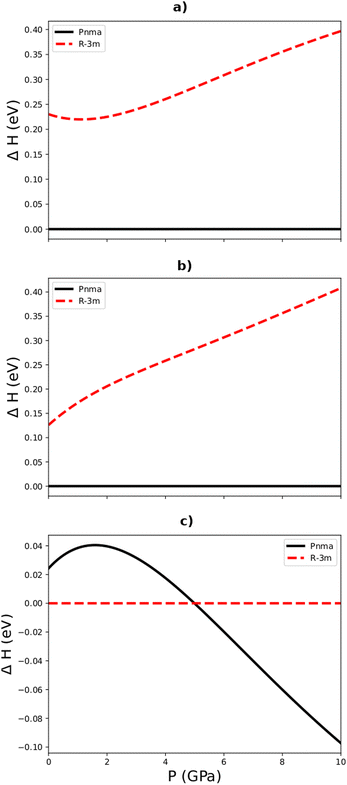 | ||
Fig. 2 Calculated relative enthalpy vs. pressure up to 10 GPa for the Pnma and R3m phases (c.f.Fig. 1) of Sb2S3 (a), Bi2S3 (b), and Sb2Se3 (c) relative to the predicted lowest-energy phase at ambient pressure, viz. the Pnma phases of Sb2S3 and Bi2S3 and the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3. m phase of Sb2Se3. | ||
In order to rule out the possibility that these results could be due to inaccuracies with the PBEsol XC functional, we also computed the enthalpies of the two phases with two additional functionals, namely the LDA37 and the PBE-D2 method.38 The results of these calculations are presented as an Appendix (Section 5.1). The unit-cell volumes of the Pnma phases obtained with the two functionals are underestimated and overestimated with respect to the PBEsol results shown in Table 1, as expected. For PBEsol, the two additional functionals predict that at ambient pressure the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 is more energetically stable than the Pnma phase, and remains so up to ∼6 GPa (LDA) and 8 GPa (PBE-D2). On the other hand, both functionals predict that the Pnma phases of Sb2S3 and Bi2S3 are more stable than the R
m phase of Sb2Se3 is more energetically stable than the Pnma phase, and remains so up to ∼6 GPa (LDA) and 8 GPa (PBE-D2). On the other hand, both functionals predict that the Pnma phases of Sb2S3 and Bi2S3 are more stable than the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase up to at least 10 GPa.
m phase up to at least 10 GPa.
For the Sb2Se3 structure, we have also investigated the effect of SoC on the energy differences between the two phases. With SoC, the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase is also predicted to be more stable than the Pnma phase with an energy difference of around 27.38 meV per f.u., a difference of around 4.67 meV per f.u. We are therefore confident that our results are not affected by the inclusion of relativistic effects.
m phase is also predicted to be more stable than the Pnma phase with an energy difference of around 27.38 meV per f.u., a difference of around 4.67 meV per f.u. We are therefore confident that our results are not affected by the inclusion of relativistic effects.
3.3 Energetic stability of the low-pressure Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m phases of Sb2Se3 at finite temperature
m phases of Sb2Se3 at finite temperature
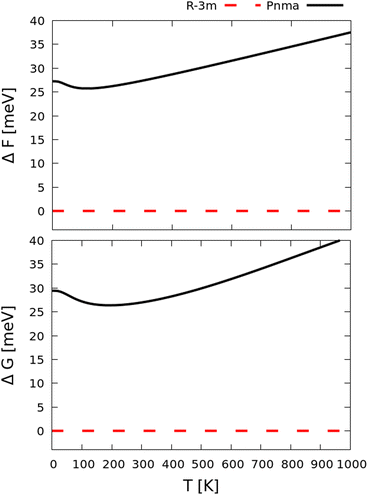
The calculations in the previous section were performed at 0 K without taking into consideration the contributions to the free energy from crystal vibrations (phonons). In order to probe whether these effects could alter the energy ordering between the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3, we performed lattice-dynamics calculations on the equilibrium and compressed structures to evaluate the constant-volume Helmholtz and constant-pressure Gibbs free energies at zero pressure (F/G; Fig. 3).
m phases of Sb2Se3, we performed lattice-dynamics calculations on the equilibrium and compressed structures to evaluate the constant-volume Helmholtz and constant-pressure Gibbs free energies at zero pressure (F/G; Fig. 3).
F is obtained by summing the lattice energy (here the DFT total energy) and the vibrational contributions to the internal energy and entropy from the zero-point atomic motion and thermal population of the harmonic phonon energy levels.70 As shown in Fig. 3, F predicts that at zero pressure the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase remains the most stable across the temperature range examined with no crossing of the free energy to suggest a transition to the Pnma phase. At 0 K, the difference in F between the two phases is 27.24 meV per f.u., which is ∼4.53 meV higher than the difference in H due to the addition of the zero-point energy (i.e. the differences in the phonon frequencies selectively stabilise the R
m phase remains the most stable across the temperature range examined with no crossing of the free energy to suggest a transition to the Pnma phase. At 0 K, the difference in F between the two phases is 27.24 meV per f.u., which is ∼4.53 meV higher than the difference in H due to the addition of the zero-point energy (i.e. the differences in the phonon frequencies selectively stabilise the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase). At 300 K, the difference between the phases shows a negligible increase from 0.11 meV per F.U. to 27.35 meV.
m phase). At 300 K, the difference between the phases shows a negligible increase from 0.11 meV per F.U. to 27.35 meV.
Another factor that can influence the ordering of two competing phases is thermal expansion. A variation of the lattice volume due to thermal expansion/contraction impacts both the lattice energy and the phonon contributions to the free energy. This can be accounted for through the quasi-harmonic approximation (QHA) where the thermal expansion of the lattice is predicted from the volume dependence of the lattice energy, phonon frequency spectrum and phonon free energy.70,71 The free energy F is computed for a series of unit-cell volumes and the equilibrium volume and Gibbs free energy G at a finite temperature T are obtained by minimizing F for a given (constant) pressure. Fig. 3 shows the difference in G between the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3. When taking into account the thermal expansion, the R
m phases of Sb2Se3. When taking into account the thermal expansion, the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase still remains stable phase with respect to the Pnma phase from 0–1000 K with a similar energy difference of 29.43 meV at 0 K to that predicted using the constant-volume F, and a slightly smaller difference of 26.96 meV at 300 K.
m phase still remains stable phase with respect to the Pnma phase from 0–1000 K with a similar energy difference of 29.43 meV at 0 K to that predicted using the constant-volume F, and a slightly smaller difference of 26.96 meV at 300 K.
To investigate the effect of pressure on the free energy, we also computed the difference in the Gibbs energy between the two phases at applied pressures from 0–5 GPa (Fig. 4). For p = 3 and 4 GPa, these calculations predict the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase to be the most energetically stable phase across the 0–1000 K temperature range examined, but it can be clearly seen that pressure reduces the energy differences between the two phases. At 4 GPa, the smallest energy difference between the two phases of ∼3.25 meV is predicted to occur between 650 and 700 K. Increasing the pressure slightly to 4.2 GPa results in the energies of the two phases becoming nearly equal at around 400 K, and at 4.3 GPa a phase transition from R
m phase to be the most energetically stable phase across the 0–1000 K temperature range examined, but it can be clearly seen that pressure reduces the energy differences between the two phases. At 4 GPa, the smallest energy difference between the two phases of ∼3.25 meV is predicted to occur between 650 and 700 K. Increasing the pressure slightly to 4.2 GPa results in the energies of the two phases becoming nearly equal at around 400 K, and at 4.3 GPa a phase transition from R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m to Pnma is predicted to occur around this temperature. At 4.5 GPa, the predicted transition temperature decreases to ∼200 K, and at 5 GPa, the Pnma phase becomes the most energetically favorable structure across the entire temperature range examined.
m to Pnma is predicted to occur around this temperature. At 4.5 GPa, the predicted transition temperature decreases to ∼200 K, and at 5 GPa, the Pnma phase becomes the most energetically favorable structure across the entire temperature range examined.
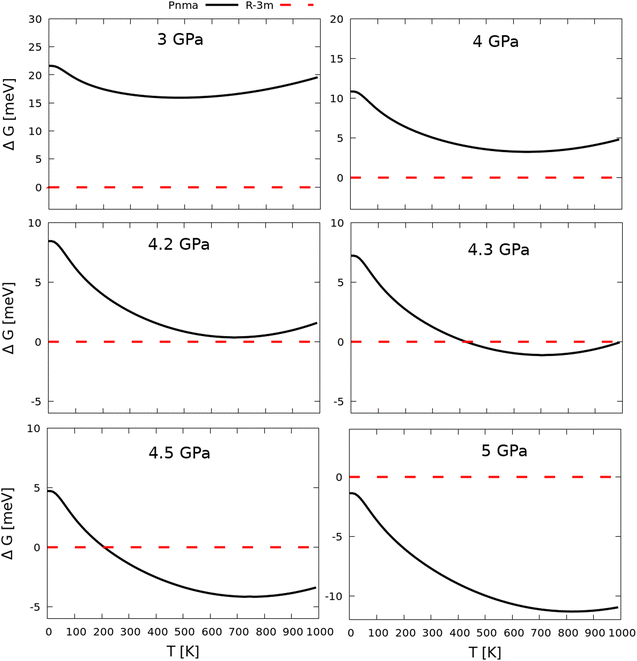 | ||
Fig. 4 Gibbs free energies of the Pnma phase of Sb2Se3 (black, solid line) relative to the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase (red, dashed line) as a function of temperature at applied pressures from 3–5 GPa. m phase (red, dashed line) as a function of temperature at applied pressures from 3–5 GPa. | ||
In summary, free-energy calculations including phonon contributions and thermal expansion at zero applied pressure also predict that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 is more stable than the Pnma phase. Interestingly, at most temperatures both F and G predict an increase in the energy difference, from which we infer that phonon contributions to the free energy selectively stabilise the R
m phase of Sb2Se3 is more stable than the Pnma phase. Interestingly, at most temperatures both F and G predict an increase in the energy difference, from which we infer that phonon contributions to the free energy selectively stabilise the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase, at least at zero applied pressure. At low finite pressures between 4.2 and 4.4 GPa, the Gibbs free energies predict a pressure-induced transition between the R
m phase, at least at zero applied pressure. At low finite pressures between 4.2 and 4.4 GPa, the Gibbs free energies predict a pressure-induced transition between the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and Pnma phases near room temperature, and predict the Pnma phase to be stable above 5 GPa, which is similar to the 4.8 GPa transition pressure predicted from the 0 K enthalpies without zero-point energy corrections (c.f. Fig. 2). The small decrease in the predicted transition pressure can be attributed to the different impacts of volume changes on the phonon spectra of the two phases.
m and Pnma phases near room temperature, and predict the Pnma phase to be stable above 5 GPa, which is similar to the 4.8 GPa transition pressure predicted from the 0 K enthalpies without zero-point energy corrections (c.f. Fig. 2). The small decrease in the predicted transition pressure can be attributed to the different impacts of volume changes on the phonon spectra of the two phases.
3.4 Dynamical stability of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m phases of Sb2Se3
m phases of Sb2Se3
Energetic stability is a necessary but not sufficient condition for a structural phase to be synthetically accessible. A second criterion is that one should also confirm the dynamical stability of the system, which can be performed by studying the phonon frequency spectrum. If imaginary frequencies are present in the phonon dispersion, this is an indication that the system is not a minimum on the structural potential-energy surface (and is instead e.g. a transition state or a hilltop in multidimensional space), and would spontaneously convert to a lower-energy structure and thus be kinetically unstable under a given set of conditions.72–77
We therefore investigated the dynamical stability of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 to confirm whether this structure could potentially be synthesized under or close to ambient conditions. To do so, we evaluated the phonon band dispersion and density of states (DoS) curves of the R
m phase of Sb2Se3 to confirm whether this structure could potentially be synthesized under or close to ambient conditions. To do so, we evaluated the phonon band dispersion and density of states (DoS) curves of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase at zero pressure and different temperatures using the QHA method described in the previous section (Fig. 5). For comparison, we also present the phonon band structure and DoS of the Pnma phase. Both structures show real frequencies across the whole of the Brillouin zone, indicating that both are dynamically stable under ambient conditions (i.e. 0 GPa and room temperature) and confirming that, as implied by the energetics calculations, both phases should be accessible under appropriate synthesis conditions.
m phase at zero pressure and different temperatures using the QHA method described in the previous section (Fig. 5). For comparison, we also present the phonon band structure and DoS of the Pnma phase. Both structures show real frequencies across the whole of the Brillouin zone, indicating that both are dynamically stable under ambient conditions (i.e. 0 GPa and room temperature) and confirming that, as implied by the energetics calculations, both phases should be accessible under appropriate synthesis conditions.
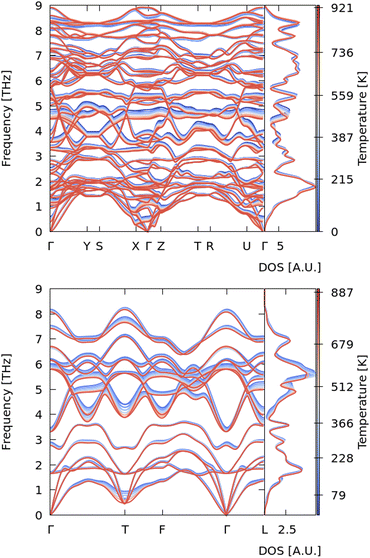 | ||
Fig. 5 Quasi-harmonic phonon dispersion curves for Pnma (top) and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Sb2Se3 (bottom). The color gradient runs from blue (low T) to red (high T) for temperatures between ∼0 and 1000 K. m Sb2Se3 (bottom). The color gradient runs from blue (low T) to red (high T) for temperatures between ∼0 and 1000 K. | ||
In this context, we note that these results are consistent with a recent theoretical study of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3, which reported phonon dispersion curves and confirmed the dynamical stability of this phase at 0 GPa and 0 K.23 This study also reported formation energies as evidence for the stability of the R
m phase of Sb2Se3, which reported phonon dispersion curves and confirmed the dynamical stability of this phase at 0 GPa and 0 K.23 This study also reported formation energies as evidence for the stability of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase. In addition, ab initio molecular dynamics calculations also confirmed that the R
m phase. In addition, ab initio molecular dynamics calculations also confirmed that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase remains structurally stable at 300 K.23
m phase remains structurally stable at 300 K.23
3.5 Mechanical stability of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m phases of Sb2Se3
m phases of Sb2Se3
Having established the energetic and dynamical stabilities of the phase R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m of Sb2Se3 at zero pressure, we also checked the mechanical (elastic) stability as a third condition that must be fulfilled for this system to be synthetically accessible. This is achieved by calculating the elastic constants and confirming that they obey the Born stability criteria when the solid is subjected to homogeneous deformations.72,78,79Table 2 lists the calculated elastic constants of the Pnma and R
m of Sb2Se3 at zero pressure, we also checked the mechanical (elastic) stability as a third condition that must be fulfilled for this system to be synthetically accessible. This is achieved by calculating the elastic constants and confirming that they obey the Born stability criteria when the solid is subjected to homogeneous deformations.72,78,79Table 2 lists the calculated elastic constants of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3 at zero pressure.
m phases of Sb2Se3 at zero pressure.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3 at zero pressure
m phases of Sb2Se3 at zero pressure
| c 11 | c 22 | c 33 | c 12 | c 13 | c 23 | c 44 | c 55 | c 66 | |
|---|---|---|---|---|---|---|---|---|---|
| Pnma | 30.92 | 81.65 | 55.20 | 17.32 | 15.10 | 26.89 | 17.83 | 25.21 | 7.69 |
| c 11 = c22 | c 33 | c 12 | c 13 = c23 | c 15 = −c25 = c46 | c 44 = c55 | c 66 | |
|---|---|---|---|---|---|---|---|
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
90.81 | 40.21 | 25.85 | 21.61 | −12.00 | 25.10 | 32.48 |
We also computed the linear compressibility of both phases using ELATE analysis tools.48 For both phases, only directions corresponding to positive linear compressibilities were obtained, indicating that both phases are mechanically stable under ambient conditions. In the case of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase, we obtained linear compressibilities between βmin = 4.9 TPa−1 (hexagonal a-axis) and βmax = 19.5 TPa−1 (hexagonal c-axis) with an anisotropy value of 3.95. For the Pnma phase, the compressibilities fall between βmin = 3.7 TPa−1 (b-axis) and βmax = 25.7 TPa−1 (a-axis) with an anisotropy of 6.87. These values are of the same order as the experimental axial compressibilities of the Pnma phase of Sb2Se3 (βa = 15.2 TPa−1, βb = 3.9 TPa−1, and βc = 8.3 TPa−1).62Table 3 summarises the elastic moduli calculated from the elastic constants, and we note that the bulk modulus of the Pnma phase of Sb2Se3 (31.8 GPa) is similar to that obtained from the Birch–Murnaghan fit (c.f.Table 1), which confirms that the elastic constants are adequately converged.
m phase, we obtained linear compressibilities between βmin = 4.9 TPa−1 (hexagonal a-axis) and βmax = 19.5 TPa−1 (hexagonal c-axis) with an anisotropy value of 3.95. For the Pnma phase, the compressibilities fall between βmin = 3.7 TPa−1 (b-axis) and βmax = 25.7 TPa−1 (a-axis) with an anisotropy of 6.87. These values are of the same order as the experimental axial compressibilities of the Pnma phase of Sb2Se3 (βa = 15.2 TPa−1, βb = 3.9 TPa−1, and βc = 8.3 TPa−1).62Table 3 summarises the elastic moduli calculated from the elastic constants, and we note that the bulk modulus of the Pnma phase of Sb2Se3 (31.8 GPa) is similar to that obtained from the Birch–Murnaghan fit (c.f.Table 1), which confirms that the elastic constants are adequately converged.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2Se3 at zero pressure obtained within the Voigt approximation using the ELATE analysis tool: bulk modulus, B0, Young's modulus, E, shear modulus, G and Poisson's ratio, υ
m phases of Sb2Se3 at zero pressure obtained within the Voigt approximation using the ELATE analysis tool: bulk modulus, B0, Young's modulus, E, shear modulus, G and Poisson's ratio, υ
| B 0 (GPa) | E (GPa) | G (GPa) | υ | |
|---|---|---|---|---|
| Pnma | 31.82 | 44.10 | 17.38 | 0.27 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
40.00 | 65.56 | 26.72 | 0.23 |
The calculated elastic constants in Table 2 fulfill the necessary and sufficient Born criteria for the mechanical stability of orthorhombic (eqn (1)) and rhombohedral (eqn (2)) systems.79 The calculated elastic constants therefore indicate that both the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and Pnma phases of Sb2Se3 are mechanically stable under ambient conditions.
m and Pnma phases of Sb2Se3 are mechanically stable under ambient conditions.
| c11, c44, c55, c66 > 0; c11c22 > c212; c11c22c33 + 2c12c13c23 − c11c223 − c22c213 − c33c212 > 0 | (1) |
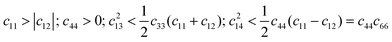 | (2) |
The elastic tensors of the Pnma phase of Sb2Se3 have previously been calculated.49 However, in these calculations, the components were overestimated as clearly shown by the comparison of the bulk modulus with experiments. Part of the disagreement could be due to the low cut-off energy used in these calculations.
In summary, our calculations on Sb2Se3 demonstrate that the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases are energetically competitive under ambient conditions and that both phases are dynamically and mechanically stable. Given that it should in principle be possible to obtain the R
m phases are energetically competitive under ambient conditions and that both phases are dynamically and mechanically stable. Given that it should in principle be possible to obtain the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase, but it has yet to be reported experimentally, it may be that the R
m phase, but it has yet to be reported experimentally, it may be that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase is difficult to form kinetically, i.e. that the Pnma phase is formed faster than the R
m phase is difficult to form kinetically, i.e. that the Pnma phase is formed faster than the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase under typical synthesis conditions. We note that the R
m phase under typical synthesis conditions. We note that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase did not appear on the pressure/temperature phase diagram prepared by Pfeiffer et al.,80 although this study did not attempt to vary the synthesis conditions at close to ambient pressure, which the present calculations suggest would allow this phase to be formed. Since our calculations increase the possibility that the R
m phase did not appear on the pressure/temperature phase diagram prepared by Pfeiffer et al.,80 although this study did not attempt to vary the synthesis conditions at close to ambient pressure, which the present calculations suggest would allow this phase to be formed. Since our calculations increase the possibility that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase could potentially be prepared under slightly non-equilibrium conditions, to help identify R
m phase could potentially be prepared under slightly non-equilibrium conditions, to help identify R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Sb2Se3 in future experiments we provide in the following section a reference structure and vibrational spectra.
m Sb2Se3 in future experiments we provide in the following section a reference structure and vibrational spectra.
3.6 Crystal structure and vibrational spectra of R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m Sb2Se3
m Sb2Se3
Table 4 lists the predicted equilibrium crystal structure (lattice parameters and atomic positions) of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Sb2Se3 obtained using the PBEsol XC functional. (The calculations with PBE + D2 and LDA yield slightly different lattice parameters, viz. PBE + D2 – a0 = 4.02 Å, c0 = 28.81 Å and V0 = 403.89 Å3; and LDA – a0 = 3.99 Å, c0 = 27.58 Å, and V0 = 381.61 Å3.) The predicted lattice parameters are in good agreement with those reported for other tetradymite-like sesquichalcogenides.81 The optimised a0 and c0 are slightly smaller than those of Bi2Se3 and much smaller than those of Sb2Te3.81
m Sb2Se3 obtained using the PBEsol XC functional. (The calculations with PBE + D2 and LDA yield slightly different lattice parameters, viz. PBE + D2 – a0 = 4.02 Å, c0 = 28.81 Å and V0 = 403.89 Å3; and LDA – a0 = 3.99 Å, c0 = 27.58 Å, and V0 = 381.61 Å3.) The predicted lattice parameters are in good agreement with those reported for other tetradymite-like sesquichalcogenides.81 The optimised a0 and c0 are slightly smaller than those of Bi2Se3 and much smaller than those of Sb2Te3.81
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3
m phase of Sb2Se3
| Site | Sym. | x | y | Z | |
|---|---|---|---|---|---|
| Sb1 | 6c | 3m | 0.00000 | 0.00000 | 0.60082 |
| Se1 | 3a | −3m | 0.00000 | 0.00000 | 0.00000 |
| Se2 | 6c | 3m | 0.00000 | 0.00000 | 0.78792 |
We have also computed the Raman and infrared (IR) spectra of the equilibrium structure to provide spectral signatures that could be used to identify the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase in experiments using routine characterisation techniques (Fig. 6).
m phase in experiments using routine characterisation techniques (Fig. 6).
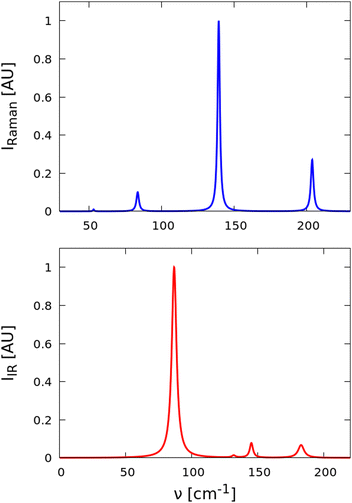 | ||
Fig. 6 Simulated Raman (top) and infrared (IR; bottom) spectra of equilibrium R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Sb2Se3. Spectral lines have been broadened with the calculated intrinsic mode linewidths at 300 K. m Sb2Se3. Spectral lines have been broadened with the calculated intrinsic mode linewidths at 300 K. | ||
The frequencies, irreducible representations and IR/Raman intensities associated with each of the zone-centre (Γ-point) vibrational modes are listed in Table 5.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of the equilibrium structure of Sb2Se3. The three acoustic modes span an irreducible representations of Γacoustic = A2u + Eu, and the remaining 12 optic modes span a representation of Γoptic = 2Eg (Raman) + 2A1g (Raman) + 2Eu (IR) + 2A2u (IR)
m phase of the equilibrium structure of Sb2Se3. The three acoustic modes span an irreducible representations of Γacoustic = A2u + Eu, and the remaining 12 optic modes span a representation of Γoptic = 2Eg (Raman) + 2A1g (Raman) + 2Eu (IR) + 2A2u (IR)
| Frequency (cm−1) | Raman intensity (105 Å4 amu−1) | IR intensity (e2 amu−1) | Spectral linewidth (cm−1) | |
|---|---|---|---|---|
| E g | 53.3 | 0.02 | Inactive | 1.02 |
| A 1g | 83.7 | 0.37 | Inactive | 2.11 |
| E u | 86.6 | Inactive | 3.47 | 4.32 |
| E u | 131.7 | Inactive | 0.03 | 3.13 |
| E g | 139.4 | 3.32 | Inactive | 1.89 |
| A 2u | 145.1 | Inactive | 0.18 | 2.94 |
| A 2u | 182.8 | Inactive | 0.29 | 5.38 |
| A 1g | 203.8 | 1.02 | Inactive | 2.16 |
The inversion symmetry in the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure leads to a mutual exclusion between the IR and Raman activities of the modes, with each spectrum being characterized by four bands.81 The most intense Raman band occurs at around 139 cm−1 (Eg), while a second prominent feature is predicted at ∼204 cm−1 (A1g). The frequency of this A1g mode is higher than that in Bi2Se3 but lower than that in In2Se3, as expected from the difference in mass between In, Sb and Bi.82,83 Lower-frequency Eg and A1g modes with much lower intensities are also found around 53 and 84 cm−1, respectively, which are again slightly blue-shifted when compared to the corresponding frequencies calculated for Bi2Se3.82 There are four IR-active modes, two with E1u symmetry (87 and 132 cm−1) and two with A2u symmetry (145 and 183 cm−1). Of these, the 87 cm−1 mode is the most prominent in the spectrum, while the second Eu mode at 132 cm−1 is comparatively weak. The two A2u bands have moderate and comparable intensities and form a pair of smaller features at higher frequencies. As expected, given the mass difference, the IR-active modes in Sb2Se3 again have slightly higher frequencies than those calculated for Bi2Se3.82
m structure leads to a mutual exclusion between the IR and Raman activities of the modes, with each spectrum being characterized by four bands.81 The most intense Raman band occurs at around 139 cm−1 (Eg), while a second prominent feature is predicted at ∼204 cm−1 (A1g). The frequency of this A1g mode is higher than that in Bi2Se3 but lower than that in In2Se3, as expected from the difference in mass between In, Sb and Bi.82,83 Lower-frequency Eg and A1g modes with much lower intensities are also found around 53 and 84 cm−1, respectively, which are again slightly blue-shifted when compared to the corresponding frequencies calculated for Bi2Se3.82 There are four IR-active modes, two with E1u symmetry (87 and 132 cm−1) and two with A2u symmetry (145 and 183 cm−1). Of these, the 87 cm−1 mode is the most prominent in the spectrum, while the second Eu mode at 132 cm−1 is comparatively weak. The two A2u bands have moderate and comparable intensities and form a pair of smaller features at higher frequencies. As expected, given the mass difference, the IR-active modes in Sb2Se3 again have slightly higher frequencies than those calculated for Bi2Se3.82
It is also worth comparing our predicted structural and vibrational properties of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Sb2Se3 to the published works pertaining to the possible synthesis of this phase. In 2013, Bera et al.25 claimed to have observed the R
m Sb2Se3 to the published works pertaining to the possible synthesis of this phase. In 2013, Bera et al.25 claimed to have observed the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 at room temperature. However, the experimental lattice parameters were not disclosed and the reported Raman spectrum is not consistent with our theoretical spectrum, although our spectrum is consistent with simulations performed in the same study. We also note that our calculations of the pressure coefficients for the Raman- and IR-active modes of tetradymite Sb2Se3 (not shown) indicate that all the modes should show positive pressure coefficients, which is again consistent with the theoretical results shown in ref. 25.
m phase of Sb2Se3 at room temperature. However, the experimental lattice parameters were not disclosed and the reported Raman spectrum is not consistent with our theoretical spectrum, although our spectrum is consistent with simulations performed in the same study. We also note that our calculations of the pressure coefficients for the Raman- and IR-active modes of tetradymite Sb2Se3 (not shown) indicate that all the modes should show positive pressure coefficients, which is again consistent with the theoretical results shown in ref. 25.
If we consider that the band gap of tetradymite-like Sb2Se3 is similar to or even larger than that of tetradymite-like Bi2Se3, we would expect the Raman spectrum of tetradymite-like Sb2Se3 to be similar. We may therefore conclude that the experimental Raman spectra reported in ref. 25 are not consistent with tetradymite-like Sb2Se3. In this vein, we note that the appearance of soft Raman modes with a negative pressure coefficient at low pressures as reported in ref. 25 is similar to those of Se and Te nano- or micro-clusters.84–87 Such features are either formed during synthesis or induced by laser heating in Raman scattering measurements at high laser powers, as has recently been discussed.87
Recently, Matetskiy et al.32 claimed to have observed the tetradymite-like phase of Sb2Se3 in MBE-deposited layers over a thick buffer layer of tetradymite-like Bi2Se3. This study reported a lattice parameter of a0 = 4.048 Å for quintuple layers of tetradymite-like Sb2Se3. The quintuple layers were observed by scanning-tunnelling microscopy (STM) to be of ∼1 nm in thickness, meaning that the c0 lattice parameter, corresponding to three quintuple layers, would be ∼30 Å. Both the reported a0 and c0 lattice parameters are consistent with our calculated values. Furthermore, the electron dispersion obtained from angle-resolved photoemission spectroscopy (ARPES) is compatible with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase. It is therefore highly probable that tetradymite-like Sb2Se3 was synthesized for the first time in this study. Unfortunately, this study did not report vibrational spectra that would allow for additional comparison to our predictions.
m phase. It is therefore highly probable that tetradymite-like Sb2Se3 was synthesized for the first time in this study. Unfortunately, this study did not report vibrational spectra that would allow for additional comparison to our predictions.
3.7 On the observation of the R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m phase of A2X3 sesquichalcogenides
m phase of A2X3 sesquichalcogenides
An analysis of bonding in layered materials under ambient conditions, including tetradymite-like BV2XVI3 and AVIBV2XVI4 chalcogenides and also transition metal dichalcogenides (TMDs), has recently been performed by plotting the van der Waals (vdW) gap spacing vs. the X–X plane spacing.88 The vdW gap spacing is defined as the interplanar distance across the interlayer space, whereas the X–X plane distance is the interplanar width across the intralayer space.88,89 In ref. 88, it was concluded from the strong correlation that TMDs show a pure interlayer vdW interaction, whereas the tetradymite-like chalcogenides do not because the vdW gap spacing is much smaller than expected for their X–X plane spacing, which is indicative of a stronger interlayer interaction than that expected for pure vdW interactions.
It has recently been suggested that the vdW gap spacings in tetradymite-like group-15 sesquichalcogenides are smaller than those found for pure vdW materials, e.g. GaSe, InSe and TMDs. This has been attributed to the presence of the extra delocalized electrons between the layers that contribute an electrostatic component to the bonding that is not present in pure vdW materials.24,90 These delocalized electrons arise from a new type of bonding, termed “metavalent bonding”, between the cations and anions inside the quintuple layers of the tetradymite-like structure, which has been demonstrated by the observation of a net charge difference between the layers in group-15 sesquichalcogenides91 and through a topological study of the electronic charge density of SnSb2Te4.92 In essence, the metavalent bonding results in the cations and anions providing extra electrons to the space between the layers, which in turn supports a stronger interlayer interaction.
In Fig. 7, we compare experimental (where available) and theoretical vdW gap spacings against X–X plane spacings for the full set of tetradymite-like BV2XVI3 (B = As, Sb, Bi; X = S, Se, Te) sesquichalcogenides.88,89 (The theoretical results are obtained with PBEsol and dispersion-corrected PBE.)
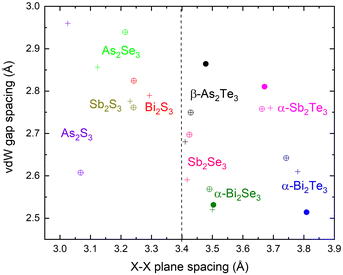 | ||
Fig. 7 Size of van der Waals gap vs. chalcogenide X–X plane spacing in the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of A2X3 sesquichalcogenides (A = As, Sb, Bi and X = Te, Se, S). The data obtained from our theoretical calculations using dispersion-corrected PBE are shown as circles with crosses. For comparison, we also show theoretical PBEsol calculations on α-Bi2Se3, α-Bi2Te3 and α-Sb2Te3 from ref. 89 (crosses) and experimental measurements on β-As2Te3, α-Bi2Se3, α-Bi2Te3 and α-Sb2Te3 from ref. 93–96 (dots). m phases of A2X3 sesquichalcogenides (A = As, Sb, Bi and X = Te, Se, S). The data obtained from our theoretical calculations using dispersion-corrected PBE are shown as circles with crosses. For comparison, we also show theoretical PBEsol calculations on α-Bi2Se3, α-Bi2Te3 and α-Sb2Te3 from ref. 89 (crosses) and experimental measurements on β-As2Te3, α-Bi2Se3, α-Bi2Te3 and α-Sb2Te3 from ref. 93–96 (dots). | ||
Fig. 7 shows that the compounds usually synthesized in the tetradymite-like structure, viz. β-As2Te3, α-Sb2Te3, α-Bi2Te3 and α-Bi2Se3, have a very small vdW gap spacings (typically below 2.9 Å) and large X–X plane spacings (typically above 3.4 Å). It is particularly striking that the Te–Te plane spacing in β-As2Te3 is below 3.5 Å, which is very different to other Te sesquichalcogenides and close to the Se–Se plane spacings in Se sesquichalcogenides such as Bi2Se3. From this analysis, we can therefore inferr that all the compounds with a stable tetradymite-like structure under ambient conditions (see Fig. 5 of ref. 88) have relatively small vdW gap spacings and X–X plane spacings between 3.4 and 3.8 Å. Since As2Se3 and Sb2Se3 do not usually crystallize in the tetradymite-like structure, it can similarly be inferred that X–X plane spacings below 3.4 Å and vdW gap spacings above 2.9 Å are outside the limit of stability for tetradymite structures of the BV2XVI3 and AVIBV2XVI4 chalcogenides. This is consistent with: (i) the locations of α-Bi2Se3 and β-As2Te3 close to the lower limit of the X–X plane spacing; (ii) the metastable tetradymite-like phase of β-As2Te3 being obtained under ambient conditions; and (iii) the metastable orthorhombic Pnma phase of Bi2Se3 (guanajuatite) being obtained under ambient conditions.17,97–99 The latter is isostructural to Bi2S3, Sb2S3 and Sb2Se3 and has been observed in high-pressure studies on α-Bi2Se3 (paraguanajuatite) on decreasing the pressure.54,69
Most importantly, the vdW gap spacings and X–X plane spacings calculated for Sb2Se3 with PBEsol and dispersion-corrected PBE are similar to those obtained for other group-15 tetradymite-like sesquichalcogenides. This lends further support to the idea that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 should be synthetically accessible with a structure similar to that predicted in our calculations. Unfortunately, despite the fact that R
m phase of Sb2Se3 should be synthetically accessible with a structure similar to that predicted in our calculations. Unfortunately, despite the fact that R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Sb2Se3 does appear to have been synthesized recently,32,88 a complete structural characterization has yet to be reported and therefore several of our calculated parameters cannot be compared to experimental measurements. We thus hope that the present study will stimulate further attempts to prepare and characterise the R
m Sb2Se3 does appear to have been synthesized recently,32,88 a complete structural characterization has yet to be reported and therefore several of our calculated parameters cannot be compared to experimental measurements. We thus hope that the present study will stimulate further attempts to prepare and characterise the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3.
m phase of Sb2Se3.
Finally, the analysis in Fig. 7 shows that all the group-15 sesquisulphides for which the theoretical R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structures have X–X plane spacings below 3.4 Å do not seem to be stable in this phase under ambient conditions according to our calculations. This is in good agreement with the high predicted enthalpies of these phases of Sb2S3 and Bi2S3. However, this does not necessarily mean that the R
m structures have X–X plane spacings below 3.4 Å do not seem to be stable in this phase under ambient conditions according to our calculations. This is in good agreement with the high predicted enthalpies of these phases of Sb2S3 and Bi2S3. However, this does not necessarily mean that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of these compounds cannot be synthesized, as R
m phase of these compounds cannot be synthesized, as R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m As2Se3 can be prepared at high pressure and high temperature, which indicates that the tetradymite-like structure of As2Se3 is metastable under ambient conditions.80,100–102 It is therefore possible that tetradymite-like As2S3, Sb2S3 and Bi2S3 could also potentially be synthesized under the right conditions.
m As2Se3 can be prepared at high pressure and high temperature, which indicates that the tetradymite-like structure of As2Se3 is metastable under ambient conditions.80,100–102 It is therefore possible that tetradymite-like As2S3, Sb2S3 and Bi2S3 could also potentially be synthesized under the right conditions.
4 Conclusions
We have performed a comparative theoretical study of the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of Sb2S3, Bi2S3, and Sb2Se3 at applied pressures of up to 10 GPa. Our calculations predict that at ambient pressure the R
m phases of Sb2S3, Bi2S3, and Sb2Se3 at applied pressures of up to 10 GPa. Our calculations predict that at ambient pressure the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (tetradymite-like) phase of Sb2Se3 is energetically more stable than the Pnma phase, in contrast to Sb2S3 and Bi2S3. This result contradicts the fact that all three compounds are usually grown on the Pnma phase. Further energetic studies of both Sb2Se3 phases show that the higher energetic stability of the R
m (tetradymite-like) phase of Sb2Se3 is energetically more stable than the Pnma phase, in contrast to Sb2S3 and Bi2S3. This result contradicts the fact that all three compounds are usually grown on the Pnma phase. Further energetic studies of both Sb2Se3 phases show that the higher energetic stability of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase with respect to the Pnma phase is predicted by three different XC functionals and is unaffected by the phonon contributions to the free energy and thermal expansion at finite temperature. Lattice dynamics and elastic tensor calculations further show that both the phases of Sb2Se3 are dynamically and mechanically stable at zero pressure. Our calculations therefore suggest that the formation of this phase should be feasible under close to ambient conditions.
m phase with respect to the Pnma phase is predicted by three different XC functionals and is unaffected by the phonon contributions to the free energy and thermal expansion at finite temperature. Lattice dynamics and elastic tensor calculations further show that both the phases of Sb2Se3 are dynamically and mechanically stable at zero pressure. Our calculations therefore suggest that the formation of this phase should be feasible under close to ambient conditions.
To aid in its identification, we have provided a theoretical crystal structure and predicted IR and Raman spectra. We have also discussed our results against the only two published works, to the best of our knowledge, that have claimed to have synthesized tetradymite-like Sb2Se3, and concluded that there is a high probability that this phase has recently been synthesized by molecular beam epitaxy on a thick buffer layer of tetradymite-like Bi2Se3.
Finally, we have discussed the stability of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure for all group-15 sesquichalcogenides by comparing the vdW gap and X–X plane spacings, which again suggests that the R
m structure for all group-15 sesquichalcogenides by comparing the vdW gap and X–X plane spacings, which again suggests that the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 should be synthetically accessible. We hope that this work will stimulate further investigation of tetradymite-like Sb2Se3 and the corresponding phases of As2Se3 and other sesquichalcogenides. These sesquichalcogenide phases could potentially show topological properties interesting for spintronics and quantum computation at moderate pressures, and also be of interest as phase change materials and highly-efficient thermoelectric materials, and for photonic devices.
m phase of Sb2Se3 should be synthetically accessible. We hope that this work will stimulate further investigation of tetradymite-like Sb2Se3 and the corresponding phases of As2Se3 and other sesquichalcogenides. These sesquichalcogenide phases could potentially show topological properties interesting for spintronics and quantum computation at moderate pressures, and also be of interest as phase change materials and highly-efficient thermoelectric materials, and for photonic devices.
5 Appendices
5.1 Enthalpy vs. pressure curves obtained using different exchange–correlation functionals
In order to verify that the variation of the enthalpy differences between the Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phases of the three sesquichalcogenides with pressure presented in Fig. 2 was not due to an issue with the PBEsol functional, we performed similar calculations using dispersion-corrected PBE (PBE-D2; Fig. 8) and the LDA (Fig. 9). For Sb2Se3 both functionals also predict the R
m phases of the three sesquichalcogenides with pressure presented in Fig. 2 was not due to an issue with the PBEsol functional, we performed similar calculations using dispersion-corrected PBE (PBE-D2; Fig. 8) and the LDA (Fig. 9). For Sb2Se3 both functionals also predict the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase to be the most energetically stable under ambient conditions. There is however some variation in the predicted transition pressure, with PBEsol and the LDA (Fig. 9) predicting similar pressures but PBE-D2 predicting a slightly higher transition point (Fig. 8).
m phase to be the most energetically stable under ambient conditions. There is however some variation in the predicted transition pressure, with PBEsol and the LDA (Fig. 9) predicting similar pressures but PBE-D2 predicting a slightly higher transition point (Fig. 8).
5.2 Electronic properties of the R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m structural phase of Sb2Se3
m structural phase of Sb2Se3
Fig. 10 shows the electronic band structure and atom- and orbital-projected (partial) density of states of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3. We have considered spin–orbit coupling effects in this calculation due to their importance in determining the electronic properties of many potential topological insulators. The valence band maximum (VBM) is mostly composed of Se p states, whereas the conduction band minimum (CBM) is a hybridization of Se and Sb p states with a small contribution from Se s states. The band gap is direct and located along the Γ–Z segment of the dispersion in the vicinity of the Γ point. Both the VBM and CBM show a “Mexican-hat” feature - indentation of the band dispersion - which indicates that our DFT+SoC calculations predict the R
m phase of Sb2Se3. We have considered spin–orbit coupling effects in this calculation due to their importance in determining the electronic properties of many potential topological insulators. The valence band maximum (VBM) is mostly composed of Se p states, whereas the conduction band minimum (CBM) is a hybridization of Se and Sb p states with a small contribution from Se s states. The band gap is direct and located along the Γ–Z segment of the dispersion in the vicinity of the Γ point. Both the VBM and CBM show a “Mexican-hat” feature - indentation of the band dispersion - which indicates that our DFT+SoC calculations predict the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 to be a topological insulator at ambient pressure. Sb2Se3 differs from similar topological insulators such as Bi2Te3104 and Bi2Se3105 for which at zero pressure this feature only occurs in the VBM but at moderate pressures the CBM also starts to develop a “Mexican-hat” feature. The calculated PBEsol band gap is around 0.23 eV. From a parabolic fitting of the band edges,103 we obtain a hole effective mass mh = −0.107 and an electron effective mass me = 0.098.
m phase of Sb2Se3 to be a topological insulator at ambient pressure. Sb2Se3 differs from similar topological insulators such as Bi2Te3104 and Bi2Se3105 for which at zero pressure this feature only occurs in the VBM but at moderate pressures the CBM also starts to develop a “Mexican-hat” feature. The calculated PBEsol band gap is around 0.23 eV. From a parabolic fitting of the band edges,103 we obtain a hole effective mass mh = −0.107 and an electron effective mass me = 0.098.
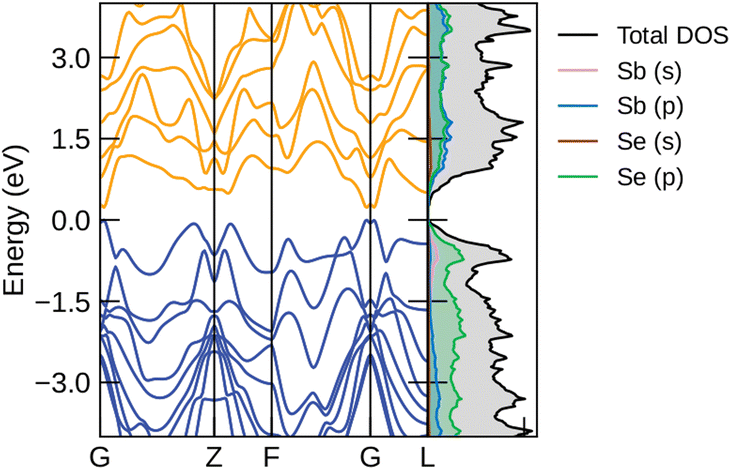 | ||
Fig. 10 Electronic band structure (left panel) and partial density of states (right panel) of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of Sb2Se3 obtained using PBEsol and including spin–orbit coupling (SoC) effects. The figure was plotted using the Python toolkit SUMO.103 m phase of Sb2Se3 obtained using PBEsol and including spin–orbit coupling (SoC) effects. The figure was plotted using the Python toolkit SUMO.103 | ||
Author contributions
E. L. d. S., M. C. S., J. M. S., P. R. H. and A. M. performed the ab initio calculations. E. L. d. S., M. C. S., J. M. S., P. R. H., A. M., D. M. G., R. V., and F. J. M. contributed to the interpretation and discussion of results. F. J. M. supervised the project. E. L. d. S., M. C. S., J. M. S., P. R. H., A. M., D. M. G., R. V., and F. J. M. contributed equally to the discussion and drafting of the paper. All authors have read and agreed to the published version of the manuscript.Data availability
Raw data from this study can be obtained from the corresponding author on reasonable request.License statement
For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is part of the MALTA Consolider Team network (RED2018-102612-T) (MINECO/AEI/10.13039/501100003329), and is supported by I + D + i project PID2019-106383GB41/42/43 (MCIN/AEI/10.13039/501100011033), by the PROMETEO/2018/123(EFIMAT) and CIPROM/2021/075 (GREENMAT) projects (Generalitat Valenciana), and by the European Union Horizon 2020 research and innovation programme under a Marie Sklodowska-Curie grant agreement (785789-COMEX). E. L. d. S., A. M., and P. R.-H. acknowledge computing time provided on the MALTA-Cluster at the University of Oviedo and on the MareNostrum facility through Red Española de Supercomputación (RES) with technical support provided by the Barcelona Supercomputing Center (QCM-2018-3-0032). E. L. d. S. also acknowledges the Network of Extreme Conditions Laboratories (NECL), financed by FCT and co-financed by NORTE 2020 through the Portugal 2020 and FEDER programmes. J. M. S. is grateful to UK Research and Innovation for the support of a Future Leaders Fellowship (MR/T043121/1) and to the University of Manchester for the previous support of a Presidential Fellowship.References
- Y. L. Chen, J. G. Analytis, J.-H. Chu, Z. K. Liu, S.-K. Mo, X. L. Qi, H. J. Zhang, D. H. Lu, X. Dai, Z. Fang, S. C. Zhang, I. R. Fisher, Z. Hussain and Z.-X. Shen, Science, 2009, 325, 178 CrossRef CAS PubMed.
- H. Zhang, C.-X. Liu, X.-L. Qi, X. Dai, Z. Fang and S.-C. Zhang, Nat. Phys., 2009, 5, 438 Search PubMed.
- M. Z. Hasan and C. L. Kane, Rev. Mod. Phys., 2010, 82, 3045 CrossRef CAS.
- J. Black, E. M. Conwell, L. Seigle and C. W. Spencer, J. Phys. Chem. Solids, 1957, 2, 240 CrossRef CAS.
- M. R. Filip, C. E. Patrick and F. Giustino, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 205125 CrossRef.
- S.-J. Sa Moon, Y. Itzhaik, J.-H. Yum, S. M. Zakeeruddin, G. Hodes and M. Gräzel, J. Phys. Chem. Lett., 2010, 1, 1524 CrossRef.
- C. E. Patrick and F. Giustino, Adv. Funct. Mater., 2011, 21, 4663 CrossRef CAS.
- Y. Zhou, L. Wang, S. Chen, S. Qin, X. Liu, J. Chen, D. Xue, M. Luo, Y. Cao, Y. Cheng, E. Sargent and J. Tang, Nat. Photonics, 2015, 9, 409 CrossRef CAS.
- L. Wang, D. Li, K. Li, C. Chen, H.-X. Deng, L. Gao, Y. Zhao, F. Jiang, L. Li, F. Huang, Y. He, H. Song, G. Niu and J. Tang, Nat. Energy, 2017, 2, 17046 CrossRef CAS.
- O. Rabin, J. Perez, J. Grimm, G. Wojtkiewicz and R. Weissleder, Nat. Mater., 2006, 5, 118 CrossRef CAS PubMed.
- K. Yao, Z. Zhang, X. Liang, Q. Chen, L.-M. Peng and Y. Yu, J. Phys. Chem. B, 2006, 110, 21408 CrossRef CAS.
- L. Cademartiri, F. Scotognella, P. O'Brien, B. Lotsch, J. Thomson, S. Petrov, N. Kherani and G. Ozin, Nano Lett., 2009, 9, 1482 CrossRef CAS.
- Mamta, Y. Singh, K. Maurya and V. Singh, Sol. Energy Mater. Sol. Cells, 2021, 230, 111223 CrossRef CAS.
- S. Chen, T. Liu, Z. Zheng, M. Ishaq, G. Liang, P. Fan, T. Chen and J. Tang, J. Energy Chem., 2022, 67, 508 CrossRef.
- Q. Li, W. Zhang, J. Peng, D. Yu, Z. Liang, W. Zhang, J. Wu, G. Wang, H. Li and S. Huang, Adv. Funct. Mater., n/a, 2112776.
- X. Ma, W. Chen, L. Tong, S. Liu, W. Dai, S. Ye, Z. Zheng, Y. Wang, Y. Zhou, W. Zhang, W. Fang, X. Chen, M. Liao and W. Gao, Opt. Laser Technol., 2021, 143, 107286 CrossRef CAS.
- Y. Kang, Q. Zhang, C. Fan, W. Hu, C. Chen, L. Zhang, F. Yu, Y. Tian and B. Xu, J. Alloys Compd., 2017, 700, 223 CrossRef CAS.
- V. Kulbachinskii, S. Buga, N. Serebryanaya, N. Perov, V. Kytin, S. Tarelkin, R. Bagramov, N. Eliseev and V. Blank, J. Phys.: Conf. Ser., 2018, 969, 012152 CrossRef.
- M. A. Tumelero, L. C. Benetti, E. Isoppo, R. Faccio, G. Zangari and A. A. Pasa, J. Phys. Chem., 2016, 120, 11797 CAS.
- N. Serebryanaya, S. Buga, R. Bagramov, I. Pahomov, N. Eliseev and V. Blank, Phys. Status Solidi B, 2020, 257, 2000145 CrossRef CAS.
- W. Liu, X. Peng, C. Tang, L. Sun, K. Zhang and J. Zhong, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 245105 CrossRef.
- W. Li, X. Wei, J.-X. Zhu, C. Ting and Y. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 89, 035101 CrossRef.
- G. Cao, H. Liu, J. Liang, L. Cheng, D. Fan and Z. Zhang, Phys. Rev. B, 2018, 97, 075147 CrossRef.
- B. J. Kooi and M. Wuttig, Adv. Mater., 2020, 32, 1908302 CrossRef CAS PubMed.
- A. Bera, K. Pal, D. V. S. Muthu, S. Sen, P. Guptasarma, U. V. Waghmare and A. K. Sood, Phys. Rev. Lett., 2013, 110, 107401 CrossRef PubMed.
- J. Ibañez, J. A. Sans, C. Popescu, J. López-Vidrier, J. J. Elvira-Betanzos, V. P. Cuenca-Gotor, O. Gomis, F. J. Manjón, P. Rodríguez-Hernández and A. Muñoz, J. Phys. Chem. C, 2016, 120, 10547 CrossRef.
- I. Efthimiopoulos, J. Zhang, M. Kucway, C. Park, R. Ewing and Y. Wang, Sci. Rep., 2013, 3, 2665 CrossRef PubMed.
- J. Anversa, S. Chakraborty, P. Piquini and R. Ahuja, Appl. Phys. Lett., 2016, 108, 212601 CrossRef.
- H. Cheng, J. Zhang, P. Yu, C. Gu, X. Ren, C. Lin, X. Li, Y. Zhao, S. Wang and Y. Li, J. Phys. Chem., 2020, 124, 3421 CAS.
- P. Kong, F. Sun, L. Xing, J. Zhu, S. Zhang, W. Li, X. Wang, S. Feng, X. Yu, J. Zhu, R. C. Yu, W. Yang, G. Shen, Y. Zhao, R. Ahuja, H. Mao and C. Jin, Sci. Rep., 2014, 4, 6679 CrossRef CAS PubMed.
- S. Das, A. Sirohi, G. Kumar Gupta, S. Kamboj, A. Vasdev, S. Gayen, P. Guptasarma, T. Das and G. Sheet, Phys. Rev. B, 2018, 97, 235306 CrossRef CAS.
- A. V. Matetskiy, V. V. Mararov, I. A. Kibirev, A. V. Zotov and A. A. Saranin, J. Condens. Matter Phys., 2020, 32, 165001 CrossRef CAS.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864 CrossRef.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2009, 102, 039902 CrossRef.
- J. P. Perdew and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 23, 5048 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188 CrossRef.
- F. D. Murnaghan, Proc. Natl. Acad. Sci. U. S. A., 1944, 30, 244 CrossRef CAS PubMed.
- F. Birch, Phys. Rev., 1947, 71, 809 CrossRef CAS.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 134106 CrossRef.
- L. Chaput, A. Togo, I. Tanaka and G. Hug, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 84, 094302 CrossRef.
- J. Skelton, L. Burton, A. Jackson, F. Oba, S. Parker and A. Walsh, Phys. Chem. Chem. Phys., 2017, 19, 12452 RSC.
- J. M. Skelton, Phonopy-spectroscopy, https://github.com/JMSkelton/Phonopy-Spectroscopy.
- A. Togo, L. Chaput and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 094306 CrossRef.
- Y. Page and P. Saxe, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 104104 CrossRef.
- R. Gaillac, P. Pullumbi and F.-X. Coudert, J. Phys.: Condens. Matter, 2016, 28, 275201 CrossRef PubMed.
- H. Koc, A. M. Mamedov, E. Deligoz and H. Ozisik, Solid State Sci., 2012, 14, 1211 CrossRef CAS.
- N. Kuganathan, E-J. Chem., 2009, 6, S147 CrossRef CAS.
- V. L. Deringer, R. P. Stoffel, M. Wuttig and R. Dronskowski, Chem. Sci., 2015, 6, 5255 RSC.
- G. P. Voutsas, A. G. Papazoglou, P. J. Rentzeperis and D. Siapkas, Z. Kristallogr., 1985, 171, 261 CAS.
- T. B. Nasr, H. Maghraoui-Meherzi, H. Abdallah and R. Bennaceur, Physica B, 2011, 406, 287 CrossRef.
- Y. Liu, K. Chua, T. C. Sum and C. Gan, Phys. Chem. Chem. Phys., 2013, 16, 345 RSC.
- L. Lundegaard, R. Miletich, T. Balic-Zunic and E. Makovicky, Phys. Chem. Miner., 2003, 30, 463 CrossRef CAS.
- I. Efthimiopoulos, C. Buchan and Y. Wang, Sci. Rep., 2016, 6, 24246 CrossRef CAS PubMed.
- P. Bayliss and W. Nowacki, Z. Kristallogr. - Cryst. Mater., 2015, 135, 308 Search PubMed.
- A. Kyono, M. Kimata, M. Matsuhisa, Y. Miyashita and K. Okamoto, Phys. Chem. Miner., 2002, 29, 254 CrossRef CAS.
- H. Koc, H. Ozisik, E. Deligöz, A. M. Mamedov and E. Ozbay, J. Mol. Model., 2014, 20, 2180 CrossRef PubMed.
- E. Zahedi and B. Xiao, Comput. Mater. Sci., 2015, 101, 301 CrossRef CAS.
- L. Lundegaard, E. Makovicky, T. Ballaran and T. Balic-Zunic, Phys. Chem. Miner., 2005, 32, 578 CrossRef CAS.
- I. Efthimiopoulos, J. Kemichick, X. Zhou, S. Khare, D. Ikuta and Y. Wang, J. Phys. Chem. A, 2014, 118, 1713 CrossRef CAS PubMed.
- J. J. Carey, J. P. Allen, D. O. Scanlon and G. W. Watson, J. Solid State Chem., 2014, 213, 116 CrossRef CAS.
- Y. Wang, M. Yanmei, G. Liu, J. Wang, Y. Li, Q. Li, J. Zhang, Y. Ma and G. Zou, Sci. Rep., 2018, 8, 14795 CrossRef PubMed.
- C. Li, J. Zhao, Q. Hu, Z. Liu, Z. Yu and H. Yan, J. Alloys Compd., 2016, 688, 329 CrossRef CAS.
- A. E. Mattsson, R. Armiento, J. Paier, G. Kresse, J. M. Wills and T. R. Mattsson, J. Chem. Phys., 2008, 128, 084714 CrossRef PubMed.
- R. Armiento and A. E. Mattsson, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 085108 CrossRef.
- A. E. Mattsson and R. Armiento, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 155101 CrossRef.
- D. Fan, J. Xu, J. Liu, Y. Li and H. Xie, High Temp. – High Pressures, 2014, 43, 351 CAS.
- M. Sternik and K. Parlinski, J. Chem. Phys., 2005, 123, 204708 CrossRef CAS.
- E. L. da Silva, J. M. Skelton, S. C. Parker and A. Walsh, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 144107 CrossRef.
- M. Born, Proc. Cambridge Philos. Soc., 1940, 36, 160 CrossRef CAS.
- M. T. Dove, Introduction to Lattice Dynamics, Cambridge University Press, 1993 Search PubMed.
- M. T. Dove, Structure and Dynamics: An Atomic View of Materials, Oxford Master Series in Physics, 2003 Search PubMed.
- M. T. Dove, Am. Mineral., 2015, 82, 213 Search PubMed.
- G. Venkataraman, Bull. Mater. Sci., 1979, 1, 129 CrossRef CAS.
- M. Di Gennaro, S. Saha and M. Verstraete, Phys. Rev. Lett., 2013, 111, 025503 CrossRef PubMed.
- B. B. Karki, G. J. Ackland and J. Crain, J. Phys.: Condens. Matter, 1997, 9, 8579 CrossRef CAS.
- F. Mouhat and F.-X. Coudert, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 224104 CrossRef.
- S. Pfeiffer, PhD thesis, Universität Stuttgart, 2009.
- F. J. Manjón, R. Vilaplana, O. Gomis, E. Pérez-González, D. Santamaría-Pérez, V. Marín-Borrás, A. Segura, J. González, P. Rodríguez-Hernández, A. Muñoz, C. Drasar, V. Kucek and V. Muñoz Sanjosé, Phys. Status Solidi B, 2013, 250, 669 CrossRef.
- R. Vilaplana, D. Santamaría-Pérez, O. Gomis, F. J. Manjón, J. González, A. Segura, A. Muñoz, P. Rodríguez-Hernández, E. Pérez-González, V. Marín-Borrás, V. Muñoz Sanjose, C. Drasar and V. Kucek, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 184110 CrossRef.
- R. Vilaplana, S. G. Parra, A. Jorge-Montero, P. Rodríguez-Hernández, A. Munoz, D. Errandonea, A. Segura and F. J. Manjón, Inorg. Chem., 2018, 57, 8241 CrossRef CAS PubMed.
- R. Vilaplana, O. Gomis, F. J. Manjón, H. M. Ortiz, E. Pérez-González, J. López-Solano, P. Rodríguez-Hernández, A. Muñoz, D. Errandonea, V. V. Ursaki and I. M. Tiginyanu, J. Phys. Chem., 2013, 117, 15773 CAS.
- V. P. Cuenca-Gotor, J. A. Sans, J. Ibáñez, C. Popescu, O. Gomis, R. Vilaplana, F. J. Manjón, A. Leonardo, E. Sagasta, A. Suárez-Alcubilla, I. G. Gurtubay, M. Mollar and A. Bergara, J. Phys. Chem., 2016, 120, 19340 CAS.
- G. P. Lindberg and B. A. Weinstein, Phys. Rev. B, 2016, 94, 134102 CrossRef.
- F. J. Manjón, S. Gallego-Parra, P. Rodríguez-Hernández, A. Muñoz, C. Drasar, V. Muñoz-Sanjosé and O. Oeckler, J. Mater. Chem. C, 2021, 9, 6277 RSC.
- R. Wang, F. R. L. Lange, S. Cecchi, M. Hanke, M. Wuttig and R. Calarco, Adv. Funct. Mater., 2018, 28, 1705901 CrossRef.
- J. Wilson and A. Yoffe, Adv. Phys., 1969, 18, 193 CrossRef CAS.
- Y. Cheng, O. Cojocaru-Mirédin, J. Keutgen, Y. Yu, M. Küpers, M. Schumacher, P. Golub, J.-Y. Raty, R. Dronskowski and M. Wuttig, Adv. Mater., 2019, 31, 1904316 CrossRef CAS PubMed.
- H. Cheng, J. Zhang, Y. Li, G. Li and X. Li, J. Appl. Phys., 2017, 121, 225902 CrossRef.
- J. A. Sans, R. Vilaplana, E. L. da Silva, C. Popescu, V. P. Cuenca-Gotor, A. Andrada-Chacón, J. Sánchez-Benitez, O. Gomis, A. L. J. Pereira, P. Rodríguez-Hernández, A. Muñoz, D. Daisenberger, B. García-Domene, A. Segura, D. Errandonea, R. S. Kumar,. Oeckler, P. Urban, J. Contreras-García and F. J. Manjón, Inorg. Chem., 2020, 59, 9900 CrossRef CAS PubMed.
- C. Morin, S. Corallini, J. Carreaud, J.-B. Vaney, G. Delaizir, J.-C. Crivello, E. B. Lopes, A. Piarristeguy, J. Monnier, C. Candolfi, V. Nassif, G. J. Cuello, A. Pradel, A. P. Goncalves, B. Lenoir and E. Alleno, Inorg. Chem., 2015, 54, 9936 CrossRef CAS PubMed.
- S. Nakajima, J. Phys. Chem. Solids, 1963, 24, 479 CrossRef CAS.
- A. Adam, Mater. Res. Bull., 2007, 42, 1986–1994 CrossRef CAS.
- T. L. Anderson and H. B. Krause, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 1307 CrossRef CAS.
- L. Vereshchagin, E. Itskevich, E. Atabaeva and S. Popova, Fiz. Tverd. Tela, 1965, 6, 2223 Search PubMed.
- E. Atabaeva, N. Bendeliani and S. Popova, Fiz. Tverd. Tela, 1973, 75, 3508 Search PubMed.
- E. Y. Atabaeva, S. A. Mashkov and S. V. Popova, Kristallografiya, 1973, 18, 173 CAS.
- L. Lityagina, L. Kulikova, I. Zibrov, T. Dyuzheva, N. Nikolaev and V. Brazhkin, J. Alloys Compd., 2015, 644, 799 CrossRef CAS.
- V. A. Kirkinsky, A. P. Ryaposov and V. G. Yakushev, in Chalcogenides of As, Sb and Bi under High Pressures, Novosibirsk: Nauka, 1985, p. 108 Search PubMed.
- V. A. Kirkinsky, A. P. Ryaposov and V. G. Yakushev, Fiz. Tverd. Tela, 1969, 11, 2382 Search PubMed.
- A. M. Ganose, A. J. Jackson and D. O. Scanlon, J. Open Source Softw., 2018, 3, 717 CrossRef.
- X. Hong, M. Newville, Y. Ding, D. Zhang, T. Irifune, G. Gu and H.-K. Mao, Phys. Rev. B, 2020, 102, 134110 CrossRef CAS.
- X. Hong, M. Newville, Y. Ding, D. Zhang, T. Irifune, G. Gu and H.-K. Mao, Phys. Rev. B, 2020, 101, 214107 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |