Doping of semicrystalline conjugated polymers: dopants within alkyl chains do it better†
Abstract
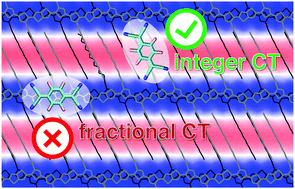
Depending on the sample preparation protocol, various structures for doped semi-crystalline polymers can be achieved, characterized by dopants either inserted in the alkyl side chains or packed with the conjugated backbone, which ultimately results in very different charge-transport and thermoelectric properties. This work targets such an intricate relationship between structure and properties with accurate hybrid quantum-classical calculations fully accounting for the effect of the environment. By considering representative structures for the crystalline domains of the F4TCNQ-doped PBTTT polymer, our calculations reveal that: (i) The electron affinity (EA) of the dopant is highly sensitive to the position occupied by the molecule in the polymer lamellae, with dopants inserted in the alkyl regions being much stronger electron acceptors than those stacked in the π-conjugated backbones (EA difference > 0.5 eV). (ii) The tiny orbital overlap between dopants in the alkyl regions and the polymer favors integer charge-transfer ground states, while dopants packed with conjugated chains are more inclined to fractional charge transfer. (iii) The Coulomb interaction between the charge carrier on the polymer and the ionized dopants is considerably (∼30%) smaller for dopants in the alkyl regions, pointing to less bound carriers. These findings rationalize the fact that record conductivities are generally associated with dopants inserted in the alkyl chains, raising awareness on the importance of controlling the dopant position in the polymer structure.
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...