A Zr-MOF nanoflower sensor and its mixed-matrix membrane for the highly sensitive detection of nitroaromatics†
Abstract
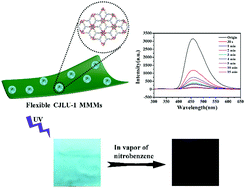
A new luminescent metal–organic framework nanoflower material CJLU-1 (Zr6(μ3-O)4(μ3-OH)4(OH)6(TCA)2(H2O)6) (H3TCA = tri-carboxylic acids 4,4′,4′′-nitrilotribenzoic acid) has been realized for the highly sensitive sensing of nitroaromatic molecules with fast response. CJLU-1 consists of six-connected Zr6 clusters and three-connected TCA ligands, and forms a 2D layered porous structure. The intrinsic 2D layered crystal structure and the optimal synthesis method create an ordered nanoflower structure constructed using ultrathin nanosheets. The highly dispersive nature and the controllable nanoflower structure with highly accessible active sites on the surface enable them to have full contact with the targeted analytes, which leads to superior sensing performance, with a high detection limit of 0.362 μM (≈83 ppb) toward 2,4,6-trinitrophenol (PA), strong anti-interference and a fast response within seconds. Moreover, a novel sensing platform, CJLU-1 mixed-matrix membranes (MMMs), is established by hybridization of the CJLU-1 nanoflower and cellulose acetate polymer, and exhibits desirable vapor sensing towards nitroaromatic molecules. This work contributes to the development of controllable nanostructured MOF probes and MOF-based MMMs as a novel sensing platform with superior mechanical strength, flexibility and sensitivity towards practical chemical sensing applications.
- This article is part of the themed collections: 2023 Journal of Materials Chemistry C Lunar New Year collection and 2022 Journal of Materials Chemistry C Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...