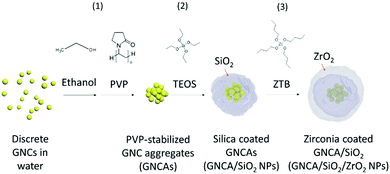
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bright and stable gold nanocluster assemblies by silica/zirconia double-shell encapsulation†
Shaochen
Zhou
 a,
Yanyan
Duan
a,
Yanyan
Duan
 b,
Kai
Liu
b,
Kai
Liu
 a and
Robin H. A.
Ras
a and
Robin H. A.
Ras
 *ac
*ac
aDepartment of Applied Physics, School of Science, Aalto University, FI-00076 Espoo, Finland. E-mail: robin.ras@aalto.fi
bIMDEA Materials Institute, Calle Eric Kandel 2, Getafe, 28906, Spain
cDepartment of Bioproducts and Biosystems, School of Chemical Engineering, Aalto University, FI-00076 Espoo, Finland
First published on 9th June 2022
Abstract
Ultrasmall gold nanoclusters are fascinating fluorescent materials with many unique properties, yet, to render them highly luminescent and stable remains challenging. In this work, solvent-induced aggregates of gold nanoclusters are encapsulated in silica/zirconia nanostructures, realizing a significantly enhanced photoluminescence efficiency and stability. These silica and zirconia coated gold nanocluster aggregates achieved a photoluminescence quantum yield of ∼55%, with high resistance to photobleaching and water, due to the stabilization by the dual-oxide matrix. Furthermore, we demonstrate their suitability for visualizing latent fingerprints.
1. Introduction
Metal nanoclusters, composed of several to dozens of atoms, emerge as promising fluorescent materials for bio/chemoanalysis,1,2 and energy3 and catalysis applications.4,5 Fluorescent gold nanoclusters have attracted most of the scientific attention due to their superior performance compared to their silver and copper counterparts.6–8 Various molecules or templates, such as amino acids,9 peptides,10 thiolates,11,12 dendrimers13 and macromolecules,1,14 have been utilized as stabilizers to synthesize gold nanoclusters with different fluorescence properties in the past decades.Nevertheless, gold nanoclusters (GNCs), like other fluorescent materials, suffer from stability problems. The ultrasmall size of GNCs results in extremely high surface activity, making them most susceptible to ambient environments. Prolonged exposure to light or heat could cause emission quenching of fluorescent GNCs. Diverse methods have been developed to improve the stability of metal nanoclusters, such as coating these ultra-small particles with protective shells,15 confining them within stable substrates16,17 and exchanging their surface ligands with robust stabilizers.18
Inert-shell coating is among the most effective ways to achieve superior stability.19,20 The shell inhibits the encapsulated substances from structural destruction or chemical degradation. However, coating usually leads to an unwanted decrease in photoluminescence quantum yields (PLQYs), which is especially unfavorable for fluorescent metal nanoclusters. Although highly luminescent metal nanoclusters (PLQY > 60%) have been reported in recent years,21,22 PLQYs of most of the metal nanoclusters remain at less than 10%. Therefore, synthetic strategies to improve both the stability and PL efficiency of GNCs are still important.
Herein, we introduce silica and zirconia coated GNC aggregates (GNCAs), namely GNCA/SiO2/ZrO2 nanoparticles (NPs), with high stability and strong emission. The GNCAs exhibit strong PL due to the aggregation-induced emission enhancement (AIEE) effect, which can offset the PLQY decrease that coating could cause to the fluorophores.23,24 However, the aggregates are extremely vulnerable to ambient conditions due to the weakly bonded structure. The outside silica–zirconia shell hereby provides a robust fixation and protection effect, leading to a significant improvement in stability. Being resistant to UV light and sensitive environments, GNCA/SiO2/ZrO2 NPs can achieve a PLQY of ∼55%, showing potential for applications like illumination, displays and fluorescence analysis.
Detection of latent fingerprints (LFPs) has been extensively applied in identity recognition. However, the LFPs could be barely seen with the naked eye because of their low optical contrast. One effective technique is to adhere powder contrast agents to the LFPs to increase the overall visibility. Various contrast agents, such as conjugated oligomers25,26 and magnetic particles,27 have been developed to visualize LFPs. The highly emissive and stable GNCA/SiO2/ZrO2 NPs can also serve as a competent fluorescent contrast agent in LFP detection, in which both the oxide matrix and incorporated GNCAs play an important role. The dual-oxide shell increases the overall stability and provides a compatible surface (e.g., fingerprint residue-affinitive), and the encapsulated GNCAs give out a strong PL that allows high-contrast imaging under UV light. Moreover, composed of low-toxicity constituents, GNCA/SiO2/ZrO2 NPs exhibit applicability in areas that require high biocompatibility.
2. Experimental
2.1 Chemicals
Gold chloride (HAuCl4·3H2O, 99%), L-glutathione reduced (GSH, ≥98%), ammonium hydroxide aqueous solution (NH3·H2O, 28–30%), and tetraethyl orthosilicate (TEOS, 98%, reagent grade) were purchased from Sigma Aldrich. Dodecane (C12H26, 99%), zirconium(IV) tert-butoxide (ZTB, 99.999% trace metal basis), and polyvinylpyrrolidone (PVP, M.W. 10000) were obtained from Alfa Aesar. Ethanol (C2H5OH, 99.5%) was purchased from Altia Oyj (Finland). All the reagents were utilized without further purification. Millipore ultrapure water with a resistance of 18.2 MΩ was used throughout the work.2.2 Synthesis of GNCs
Fluorescent gold nanoclusters are synthesized according to reported procedures.10 0.5 mL of HAuCl4 aqueous solution (20 mM) is mixed with 4.35 mL of ultrapure water, followed by the addition of GSH aqueous solution (100 mM, 150 μL). The as-obtained solution is stirred at 20 °C for 15 min until it becomes colorless and transparent, which is then heated at 70 °C for another 24 h. Byproducts are removed after 12 h of dialysis to purify the as-obtained GNC solution, which is stored under 4 °C for subsequent use.2.3 Synthesis of GNCA/SiO2 NPs
GNCAs are encapsulated into silica matrices using a modified Stöber method. 1 mL of GNC solution is mixed with 9 mL of ethanol to form dense GNCAs, followed by the addition of 200 mg of PVP. The mixture is ultrasonicated at 37 kHz, 100 W for 10 min. Then, NH3·H2O solution (125 μL) is added into the system, followed by slow addition of TEOS (250 μL) to initiate the hydrolysis and condensation of silane. The system is stirred at 400 rpm, 20 °C for 72 h. The pale-yellow products are collected by centrifugation at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, followed by washing with ethanol to remove excess reactants and byproducts. The final products are obtained after three-time washing and calcination at 50 °C for 12 h.
000 rpm for 10 min, followed by washing with ethanol to remove excess reactants and byproducts. The final products are obtained after three-time washing and calcination at 50 °C for 12 h.
2.4 Synthesis of GNCA/SiO2/ZrO2 NPs
Typically, 100 mg of the as-prepared GNCA/SiO2 NPs are dispersed in 10 mL of dodecane after sonication at 37 kHz, 100 W for 20 min. 100 μL of ZTB is added into the dispersion, which is stirred without sealing at 400 rpm, 20 °C for 48 h. The hydrolysis and condensation of ZTB proceed spontaneously and consume the residual water in the air. The products are collected by centrifugation at 6000 rpm for 5 min, which are washed with ethanol three times. GNCA/SiO2/ZrO2 NPs are obtained after calcination at 50 °C for 12 h.2.5 Characterization
Micromorphology is analyzed by high-resolution transmission electron microscopy (HR-TEM) and scanning electron microscopy (SEM), using a JEM 2800 HR-TEM and a JSF 7500FA high-resolution analytical SEM, respectively. HR-TEM is performed at an acceleration voltage of 200 kV. The samples for the TEM measurement are prepared by drop-casting on holey carbon copper grids. UV-vis absorption spectra are collected using a UV-vis-NIR Agilent Cary 5000 spectrophotometer. Fourier transmission infrared spectroscopy (FTIR) is performed using a Nicolet 380 FTIR spectrometer (Thermo Electron Corporation). Dynamic light scattering (DLS) analysis is carried out using a Malvern Zetasizer Nano-ZS90 instrument. Steady-state fluorescence spectroscopy is performed using a PTI QuantaMaster 40 fluorescence spectrometer. Time-resolved fluorescence spectroscopy and absolute photoluminescence quantum yield (PLQY) measurements are carried out with an FS5 Spectrofluorometer (Edinburgh Instruments). PLQYs are measured using the SC-30 module coupled with an integrating sphere, in which the powder or liquid samples are excited at 390 nm. The PL decay curves are collected and analyzed using a time correlation single photon counting (TCSPC) system. A picosecond pulsed diode laser (EPL-375, Edinburgh Instruments) with a pulse width of 64.3 ps and a wavelength of 377.6 nm is used for excitation.2.6 Latent fingerprint detection
The latent human fingerprints are acquired by pressing for 5 s on clean substrates (glass, polystyrene and aluminum alloy). The GNCA/SiO2/ZrO2 powder is then introduced on the substrates to cover the imprinted area, which is quickly adhered to the LFPs. The excess powder is removed by a gentle N2 gas flow. The pictures are taken by a phone camera under room light and UV light at 365 nm, respectively.3. Results and discussion
3.1 Synthesis of GNCA/SiO2/ZrO2 NPs and their optical properties
The as-prepared GNCs are Au(0) clusters that are capped by Au(I)–glutathione oligomeric complexes, whose emission originates from charge transfer within their Au(I) rich surface.10 Reducing the distance between the GNCs will lead to a substantial increase in the fluorescence intensity because of the AIEE effect, which is effective in boosting the PL efficiency of various fluorescent metal nanoclusters.28–30
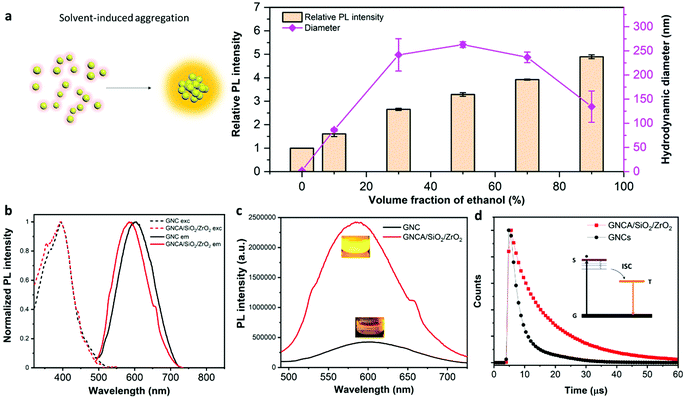
It is thermodynamically favorable for these polar GNCs to aggregate in the ethanol–water environment, due to the disruption of the hydration shell and charge neutralization of GNCs.23Fig. 1(a) shows the hydrodynamic diameter and relative PL intensity of the GNC water–ethanol mixture, which change with the volume fraction of ethanol. The hydrodynamic diameter of the dispersed phase increases from initial ∼2.5 nm to ∼262 nm as the volume fraction of ethanol increases to 50%, which indicates large aggregation of GNCs. The hydrodynamic diameter decreases further when more ethanol is present, reaching ∼134 nm when the volume of ethanol accounts for 90%. The PL emission also changes with the clustering of GNCs. Aggregation of GNCs leads to a constant increase in the emission intensity, as well as a blue shift in the peak wavelength. The emission intensity increases by 389%, and the peak blue-shifts from 603 nm to 589 nm (Fig. S1, ESI†) upon the formation of GNCAs (ethanol 90 v/v%). Clearly, the clustering of GNCs decreases the average inter-particle distance and gives rise to the AIEE effect.
PVP molecules are added to stabilize the nanocluster aggregates from precipitation by concentrating on the interface between the ethanol and GNC aqueous droplets because of their amphiphilic properties.31 PVP not only keeps the GNCAs dispersed in ethanol, but also directs the hydrolysis and condensation of silane around the aggregates. GNCAs are thereby encapsulated in a SiO2 matrix. It is noteworthy that the formation of silica not only provides a protective layer for the GNCAs, but also consumes most of the water molecules in the droplet, which is necessary for the subsequent zirconia coating. The ZrO2 precursor, ZTB, is too water-sensitive to hydrolyze controllably in a water-rich environment. Thus, zirconia is deposited on the silica coated GNCAs in an oil phase. ZTB reacts with the ambient water and produces a ZrO2 layer that covers the silica surface, producing GNCA/SiO2/ZrO2 NPs.
Fig. 1(c) shows the emission brightness comparison between GNCA/SiO2/ZrO2 NPs and GNCs. Both the emission spectra and inset picture reveal that GNCA/SiO2/ZrO2 NPs produce a much brighter emission than the GNCs do. GNCA/SiO2/ZrO2 NPs have an absolute PLQY of 54.79%, an approximately eleven-fold increase with respect to 4.76% of GNCs (Table S1, ESI†). It is noteworthy that coating could in principle lead to a decrease in the PLQY, when the outside layer could not only cause an emission energy loss, but also increase the light absorption. In addition, the aggregation of fluorophores could also result in the loss of PL (aggregation-induced quenching), due to the depopulation of excitons.33,34 Yet, in our work, we managed to offset these negative effects by taking advantage of the AIEE properties.
The AIEE origin of the glutathione-capped metal nanoclusters has been discussed in recent studies.10,35,36 Structure rigidification is one of the major causes for the conspicuous emission enhancement.21 In this work, GNCAs in the oxide matrixes are most likely affected by the structure rigidification triggered by aggregation and matrix encapsulation, because of which the non-irradiative recombinations are greatly inhibited.
As discussed above, the PL of GNCs stems from the radiative ligand–metal charge transfers. The ligand–metal charge transfer would go through non-radiative pathways when there are fierce thermal motions, including the nanocluster movement and ligand fluctuation, to meet the demand for thermal energy. The thermal motions decrease the PLQYs and PL lifetimes of GNCs profoundly, since fewer excitons go through the radiative pathway for the recombination.21,37 This can be partially manifested by the significantly stronger PL intensities of GNCs and GNCA/SiO2/ZrO2 NPs at low temperatures (Fig. S2 and S3, ESI†).
The aggregation extent and the encapsulation in the oxide matrixes provide a quite rigid medium for every encapsulated GNC. The GNCs are sterically confined, and their surface ligands are given little freedom for rotation, fluctuation, and other thermal energy-consuming motions. The fewer thermal motions, the fewer non-radiative ligand–metal charge transfers. Therefore, more excitons are going through the radiative pathways for the recombination, increasing the PLQYs and the PL lifetimes. This explains the great PL performance enhancement that is observed in GNCA/SiO2/ZrO2 NPs as compared to that of GNCs.
3.2 Morphology, composition and structure
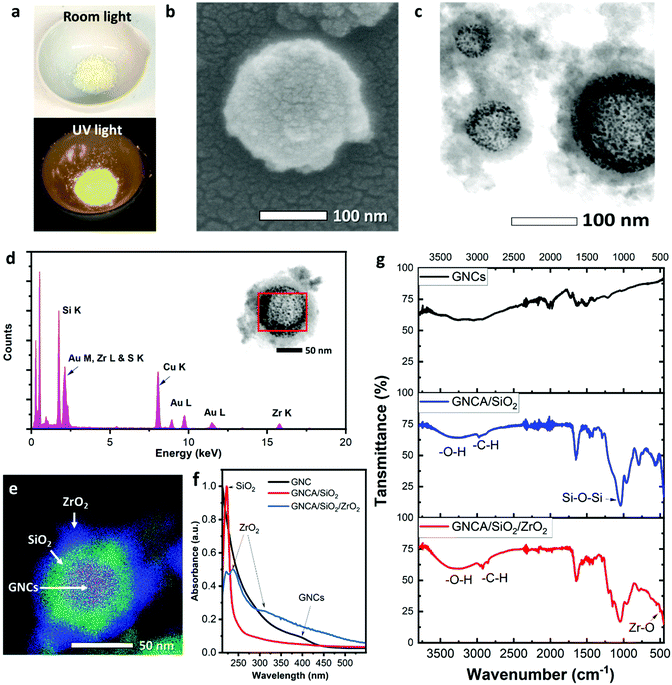
Fig. 2(f) shows the UV-vis absorption spectra of GNCs, GNCA/SiO2 NPs and GNCA/SiO2/ZrO2 NPs. Compared with GNCs, GNCA/SiO2 NPs show a dominant feature of silica. The characteristic absorption peak of GNCs at 396 nm10 becomes inconspicuous in the absorption spectrum of GNCA/SiO2 NPs. But the absorption peak at 223 nm, the characteristic of SiO2,38 is intense. The spectrum of GNCA/SiO2/ZrO2 NPs shows other apparent absorption peaks at 237 nm and 309 nm, which are assigned to ZrO2,39 besides the peak at 223 nm for the SiO2 component. This result further confirms the dual-oxide feature of GNCA/SiO2/ZrO2 NPs. Plasmonic absorption peaks at 500–550 nm for plasmonic gold NPs are absent in all the spectra, demonstrating that no larger gold particles have been formed after the silica and zirconia coating.
Fig. 2(g) shows the FTIR spectra of GNCs, GNCA/SiO2 NPs and GNCA/SiO2/ZrO2 NPs. It should be noted that the spectrum of GNCA/SiO2/ZrO2 NPs is quite similar to that of GNCA/SiO2 NPs, but different from that of GNCs, since most of the transmittance peaks stemming from the SiO2 component. The peak at 1071 cm−1 is ascribed to the asymmetric stretching vibration of Si–O–Si, a typical bond in silica. The peaks at 553 and 502 cm−1 are assigned to the stretching vibration of Zr–O,40 a typical bond in zirconia. It is worth mentioning that the characteristic peaks of Zr–O bonds are concentrated in the range of 400–800 cm−1. As a result, the transmittance band of GNCA/SiO2/ZrO2 NPs exhibits a stronger intensity than that of GNCA/SiO2 NPs in this wavenumber range (Fig. S9, ESI†). In general, all the spectral results indicate that the GNCAs are well encapsulated in the SiO2/ZrO2 matrix.
3.3 Stability evaluation
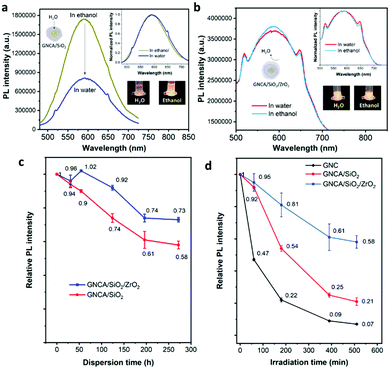
Stability is of great significance to practical applications for fluorescent metal nanoclusters. Fig. 3 reveals the emission changes of GNCA/SiO2/ZrO2 NPs under different conditions, such as exposure to water and UV light. The higher resistance to PL changes indicates a better stability for the GNCAs in the dual-oxide matrix.Fig. 3(c) shows the relative PL intensity change of GNCA/SiO2 NPs and GNCA/SiO2/ZrO2 NPs in water to reveal their sustainability in water resistance. The emission intensity of GNCA/SiO2 NPs shows a gradual decrease in the intensity, which is ∼58% of the initial value after dispersion for 272 h. However, the emission intensity of GNCA/SiO2/ZrO2 NPs decreases more slowly in the first 124 h, which decreases by only ∼8% and remains at ∼73% of the initial value after 272 h in water. These results indicate better hydrostability for GNCA/SiO2/ZrO2 NPs as compared to that of GNCA/SiO2 NPs. It is the SiO2/ZrO2 shell that keeps most of the water molecules away, and maintains the aggregates from collapsing to discrete, weakly emissive GNCs.
A secondary coating with zirconia can improve the photostability conspicuously. The emission intensity of GNCA/SiO2/ZrO2 NPs remains at ca.60% of the initial value after 510 min under high-intensity UV light, significantly higher than those of GNCs and GNCA/SiO2 NPs. Obviously, the SiO2/ZrO2 shell is more effective in inhibiting the unwanted photochemical reactions by both preventing active species (e.g., photo-generated free radicals) and prohibiting the mobility of GNCs. Varying the amount of zirconia results in a significant change in photostability: more zirconia leads to a better UV resistance (Fig. S11 and Table S2, ESI†). These results suggest the indispensability of ZrO2 in the photostability improvement.
3.4 Latent fingerprint detection
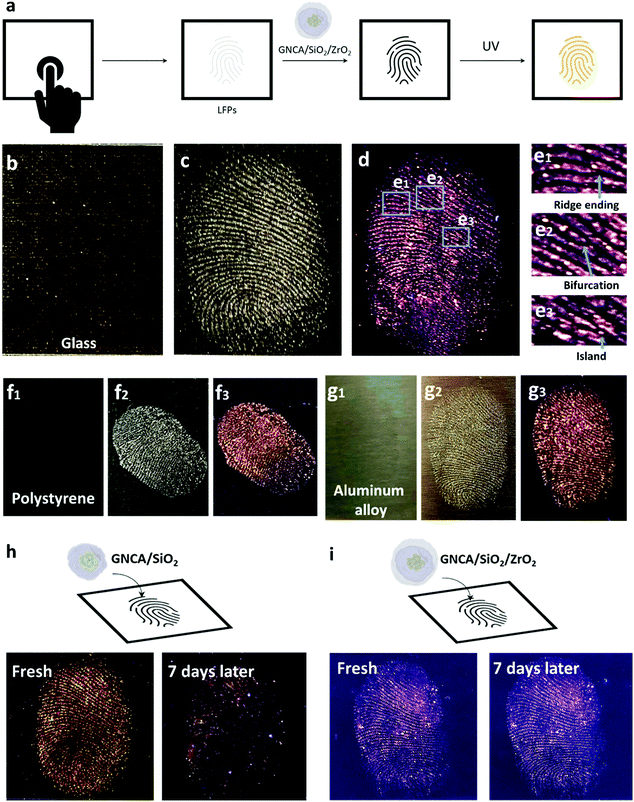
As shown in Fig. 4, GNCA/SiO2/ZrO2 NPs can be applied as an effective contrast agent in imaging the LFPs, which cannot be easily found on many surfaces due to the low-optical contrast. Detection of LFPs requires a strong adhesion between the contrast agent and fingerprint residues to achieve an easy visualization. Besides, a significant PLQY is also necessary to ensure a clear, emissive fingerprint pattern for detailed recognition under light excitation. Composed of highly luminescent GNCAs and the fingerprint residue-affinitive oxide shell, GNCA/SiO2/ZrO2 NPs show promise in LFP detection. Fig. 4(b) and (c) display the photographs of a LFP on the glass substrate before and after adhesion by the GNCA/SiO2/ZrO2 NPs. The fingerprint is almost invisible on the transparent substrate, which emerges after adhesion by the GNCA/SiO2/ZrO2 powder. Fingerprint details, such as ridge flows and ridge orientation field, can be clearly seen under visible light (Fig. 4(c)). The fingerprint becomes even clearer under UV excitation, as a result of a larger contrast between the emissive pattern and the dark background (Fig. 4(d)). Fig. 4(e1)–(e3) reveal more morphological details. A ridge ending, a bifurcation and an island can be observed in the marked areas. And a right loop pattern can be found in the core area of the fingerprint. All the morphological details developed by the GNCA/SiO2/ZrO2 powder provide sufficient information for fingerprint type classification and indexing.41More substrates are used to investigate the compatibility of the GNCA/SiO2/ZrO2 powder in LFP detection. A transparent, hydrophobic polystyrene cover and a hydrophilic aluminum alloy plate are selected as the typical substrates with different hydrophilicities and transparency. As shown in Fig. 4(f) and (g), LFPs on these substrates can be easily visualized by the GNCA/SiO2/ZrO2 powder, demonstrating good compatibility with various substrates.
Interestingly, the LFP patterns developed by GNCA/SiO2/ZrO2 NPs exhibit better storage stability than those by GNCA/SiO2 NPs. As shown in Fig. 4(h), the pattern developed by GNCA/SiO2 NPs gives out a greatly quenched PL after a seven-day storage in the dark environment (temperature: 21 °C; relative humidity: 80%). As a result, the background interference becomes strong, increasing the difficulty in further analysis. However, the emissive pattern developed by GNCA/SiO2/ZrO2 NPs shows little change after 7 days (Fig. 4(i)), suggesting better resistance to the ambient environment. A denser SiO2/ZrO2 shell is responsible for the stability difference between GNCA/SiO2/ZrO2 NPs and GNCA/SiO2 NPs.
Conclusions
We report a facile method to encapsulate GNC aggregates obtained from a solvent-induced assembly process into SiO2/ZrO2 NPs. The GNC aggregates give out a strong emission, which are stabilized and rigidified by the inert dual-oxide shell. Importantly, this synthetic strategy improves both the luminescence and stability of the fluorescent GNCs at the same time, which has always been a great challenge. The zirconia–silica–GNC nanocomposite is found to be a good fluorescent contrast agent in visualizing the latent fingerprints. The oxide shell provides a compatible surface that can easily adhere to fingerprint residues on diverse substrates, and the encapsulated GNC aggregates, which emit a bright PL to reveal the morphological details of fingerprints under light excitation. In summary, this work provides an effective way to obtain biofriendly, stable, high-performance metal nanocluster composites, which exhibit promise in various applications such as analysis, illumination, displays and catalysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was carried out under the Academy of Finland Center of Excellence Program (2022-2029) in Life-Inspired Hybrid Materials (LIBER) (project number 346109), Academy Project (310799) and Academy Postdoctoral Research Grant (13332370). We also acknowledge the provision of facilities and technical support by the OtaNano Nanomicroscopy Center of Aalto University (Aalto NMC).References
- J. Liu, TrAC, Trends Anal. Chem., 2014, 58, 99 CrossRef CAS.
- L. Shang, S. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401 CrossRef CAS.
- A. Mathew and T. Pradeep, Part. Part. Syst. Charact., 2014, 31, 1017 CrossRef CAS.
- H. Kawasaki, H. Yamamoto, H. Fujimori, R. Arakawa, M. Inada and Y. Iwasaki, Chem. Commun., 2010, 46, 3759 RSC.
- X. Du and R. Jin, ACS Nano, 2019, 13, 7383 CrossRef CAS PubMed.
- R. Jin, Y. Zhu and H. Qian, Chem. – Eur. J., 2011, 17, 6584 CrossRef CAS PubMed.
- A. Cantelli, G. Guidetti, J. Manzi, V. Caponetti and M. Montalti, Eur. J. Inorg. Chem., 2017, 5068 CrossRef CAS.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346 CrossRef CAS PubMed.
- X. Yang, M. Shi, R. Zhou, X. Chen and H. Chen, Nanoscale, 2011, 3, 2596 RSC.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662 CrossRef CAS PubMed.
- Z. Gan, Y. Lin, L. Luo, G. Han, W. Liu, Z. Liu, C. Yao, L. Weng, L. Liao and J. Chen, Angew. Chem., Int. Ed., 2016, 55, 11567 CrossRef CAS PubMed.
- R. R. Nasaruddin, T. Chen, N. Yan and J. Xie, Coord. Chem. Rev., 2018, 368, 60 CrossRef CAS.
- J. Zheng, C. Zhang and R. M. Dickson, Phys. Rev. Lett., 2004, 93, 077402 CrossRef PubMed.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS PubMed.
- X. Le Guével, B. Hötzer, G. Jung and M. Schneider, J. Mater. Chem., 2011, 21, 2974 RSC.
- G. De Cremer, B. F. Sels, J. Hotta, M. B. Roeffaers, E. Bartholomeeusen, E. Coutiño-Gonzalez, V. Valtchev, D. E. De Vos, T. Vosch and J. Hofkens, Adv. Mater., 2010, 22, 957 CrossRef CAS PubMed.
- A. Royon, K. Bourhis, M. Bellec, G. Papon, B. Bousquet, Y. Deshayes, T. Cardinal and L. Canioni, Adv. Mater., 2010, 22, 5282 CrossRef CAS PubMed.
- M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C.-T. Dinh, J. D. Padmos, K. Ayoo, P. J. Garrett, M. Nambo, J. H. Horton, E. H. Sargent, H. Häkkinen, T. Tsukuda and C. M. Crudden, Nat. Chem., 2019, 11, 419 CrossRef CAS PubMed.
- F. Zhang, Z.-F. Shi, Z.-Z. Ma, Y. Li, S. Li, D. Wu, T.-T. Xu, X.-J. Li, C.-X. Shan and G.-T. Du, Nanoscale, 2018, 10, 20131 RSC.
- X. Li, W. Cai, H. Guan, S. Zhao, S. Cao, C. Chen, M. Liu and Z. Zang, Chem. Eng. J., 2021, 419, 129551 CrossRef CAS.
- K. Pyo, V. D. Thanthirige, K. Kwak, P. Pandurangan, G. Ramakrishna and D. Lee, J. Am. Chem. Soc., 2015, 137, 8244–8250 CrossRef CAS PubMed.
- X. Le Guével, C. Spies, N. Daum, G. Jung and M. Schneider, Nano Res., 2012, 5, 379 CrossRef.
- X. Wu, L. Li, L. Zhang, T. Wang, C. Wang and Z. Su, J. Mater. Chem. B, 2015, 3, 2421 RSC.
- T. Kim, H. Jang, S. Kim, J.-H. Lee, S.-Y. Kim, N. L. Jeon, J. M. Song and D.-H. Min, Langmuir, 2018, 34, 173 CrossRef CAS PubMed.
- S. Zhang, R. Liu, Q. Cui, Y. Yang, Q. Cao, W. Xu and L. Li, ACS Appl. Mater. Interfaces, 2017, 9, 44134 CrossRef CAS PubMed.
- Z. Soran-Erdem, T. Erdem, K. Gungor, J. Pennakalathil, D. Tuncel and H. V. Demir, ACS Nano, 2016, 10, 5333 CrossRef CAS PubMed.
- P. Hazarika, S. M. Jickells, K. Wolff and D. A. Russell, Angew. Chem., Int. Ed., 2008, 47, 10167 CrossRef CAS PubMed.
- X. Gao, Y. Lu, M. Liu, S. He and W. Chen, J. Mater. Chem. C, 2015, 3, 4050 RSC.
- K. Zheng, X. Yuan, K. Kuah, Z. Luo, Q. Yao, Q. Zhang and J. Xie, Chem. Commun., 2015, 51, 15165 RSC.
- Z. Wang, B. Chen, M. Zhu, S. V. Kershaw, C. Zhi, H. Zhong and A. L. Rogach, ACS Appl. Mater. Interfaces, 2016, 8, 33993 CrossRef CAS PubMed.
- J. Lee, Macromol. Biosci., 2005, 5, 1085 CrossRef CAS PubMed.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568 CrossRef CAS PubMed.
- A. Haugeneder, U. Lemmer and U. Scherf, Chem. Phys. Lett., 2002, 351, 354 CrossRef CAS.
- J. Gierschner, H.-J. Egelhaaf, D. Oelkrug and K. Müllen, J. Fluoresc., 1998, 8, 37 CrossRef CAS.
- M. Wu, J. Zhao, D. M. Chevrier, P. Zhang and L. Liu, J. Phys. Chem. C, 2019, 123, 6010 CrossRef CAS.
- X. Jia, J. Li and E. Wang, Small, 2013, 9, 3873 CrossRef CAS PubMed.
- Q. Li, M. Zhou, W. Y. So, J. Huang, M. Li, D. R. Kauffman, M. Cotlet, T. Higaki, L. A. Peteanu, Z. Shao and R. Jin, J. Am. Chem. Soc., 2019, 141, 5314 CrossRef CAS PubMed.
- Y. Yusof and M. R. Johan, CrystEngComm, 2014, 16, 8570 RSC.
- S. Mallick, S. Rana and K. Parida, Dalton Trans., 2011, 40, 9169 RSC.
- K. Gurushantha, K. S. Anantharaju, L. Renuka, S. C. Sharma, H. P. Nagaswarupa, S. C. Prashantha, Y. S. Vidya and H. Nagabhushana, RSC Adv., 2017, 7, 12690 RSC.
- D. Maltoni, D. Maio, A. K. Jain and S. Prabhakar, Handbook of fingerprint recognition, Springer Science & Business Media, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc00824f |
| This journal is © The Royal Society of Chemistry 2022 |