White-light defect emission and enhanced photoluminescence efficiency in a 0D indium-based metal halide†
Abstract
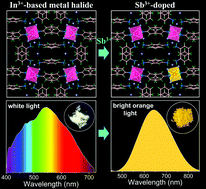
Low-dimensional ns2-metal halide compounds with confined structures and localized electronic states have received considerable attention due to their superior luminescence properties, while the intriguing photophysical dynamics of metal halides without ns2 electrons is still elusive and has yet to be investigated. Herein, we have developed a novel In3+-based (C6H8N)6InBr9 single crystal that exhibits intrinsic white-light emission at room temperature. Such white light stems from multiple defect states owing to the presence of Br vacancies, which however delivers a low photoluminescence quantum yield (PLQY) (2.72%) due to a severe thermal quenching effect. To improve the photoluminescence efficiency, the Sb3+ ion with a 5s2 lone pair has been embedded into the lattice, which leads to broadband orange emission with a high PLQY of up to 71.84%. The enhanced PL efficiency results from efficient triplet self-trapped excitons (STEs) in the (SbBr6)3− octahedron and the energy transfer from defect states to STE states. This work not only sheds light on the mechanism of defect-related white-light emission, but provides a successful strategy for designing novel materials with excellent PL properties for versatile optical applications.
- This article is part of the themed collection: Editor’s choice collection: luminescent metal halides


 Please wait while we load your content...
Please wait while we load your content...