Open Access Article
Open Access ArticleModular chiral Eu(III) complexes for efficient circularly polarized OLEDs†
Francesco
Zinna
 *a,
Lorenzo
Arrico
*a,
Lorenzo
Arrico
 a,
Tiziana
Funaioli
a,
Lorenzo
Di Bari
a,
Tiziana
Funaioli
a,
Lorenzo
Di Bari
 *a,
Mariacecilia
Pasini
*a,
Mariacecilia
Pasini
 b,
Chiara
Botta
b and
Umberto
Giovanella
b,
Chiara
Botta
b and
Umberto
Giovanella
 *b
*b
aDipartimento di Chimica e Chimica Industriale, Università di Pisa, via Moruzzi 13, 56124, Pisa, Italy. E-mail: francesco.zinna@unipi.it; lorenzo.dibari@unipi.it
bCNR-SCITEC via A. Corti 12, 20133, Milano, Italy. E-mail: umberto.giovanella@scitec.cnr.it
First published on 14th December 2021
Abstract
Achieving both high dissymmetry factors and strong emission in circularly polarized (CP) luminescent materials and, at the same time, compatibility with manufacturing processes for organic electronic devices, is a crucial issue for reliable applications of CP emitters in many fields, such as chiral electronics and optoelectronics. In this communication, we show that the independent choice of the sensitizing and the chirality inducing ligands allows europium(III) complexes to meet the multiple requirements for solution processed efficient CP electroluminescent devices.
Materials emitting circularly polarized (CP) luminescence1–5 are attracting multidisciplinary interest, as they open the way to various applications in the field of enantiospecific (bio)assays, telecommunication, spin-selective devices, etc.6,7
In the context of chiral electronics and optoelectronics, in the last few years, several research groups have explored the possibility of obtaining CP electroluminescence (EL) from Organic Light-Emitting Diodes (OLED) by employing chiral (non-racemic) emitters in the active layer.2,8 Such devices are promising for applications in 3D panels, more efficient anti-glare displays, as well as in optical data storage and encryption.9
Several successful examples of CP-OLEDs have been reported in recent years, in which CP-EL is obtained through different approaches,1,9i.e. by using single organic molecules,10 d11 or f12,13 metal complexes, or supramolecular systems.14 On the other hand, several challenges, often difficult to meet all at the same time, remain open: (i) maximizing EL efficiency, (ii) maximizing the circular polarization output (quantified by the dissymmetry factor g), (iii) low-cost device manufacturing with extended stability, (iv) availability of the chiral emitters at the required scale.
Lately, our groups have investigated the use of chiral lanthanide complexes (in particular, Europium) as intrinsic chiral emitters in the active layer of OLEDs, obtaining remarkable results in terms of EL dissymmetry factor (gel = 2(IL − IR)/(IL + IR), IL and IR being the left and right CP component of EL).12,13 Indeed, using chiral lanthanide emitters offers several advantages, such as high dissymmetry factors, high photoluminescence (PL) quantum yield (PLQY) with narrow bands, possibility of deriving effective ligands from the natural chiral pool and facile compound preparation.15 Another feature offered by lanthanide systems are bands with opposite polarization, which may be desirable in certain applications, such as near-field multiplexed communications. On the other hand, extracting high EL efficiency from lanthanide-based CP-OLEDs still remains a challenge. A possible solution to these issues can be found in the coordination chemistry of lanthanides. In fact, the high coordination number of lanthanides and the possibility of preparing heteroleptic complexes16 allow one to tune the desired properties by using a modular approach.
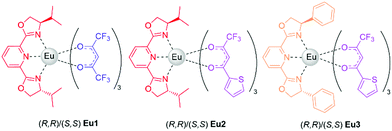
Indeed, different ligands, each performing a different role, can be chosen thus tuning the required features (partially) independently one from the other. For our purposes, the ligands must fulfil two essential functions: an efficient luminescence sensitization (antenna effect) ensuring, as well, a good radiative exciton recombination, and an effective chiral induction. In this work, we manufacture and investigate CP-OLEDs devices embedding three different Eu(III) complexes (hereafter called Eu1–3, Scheme 1) obtained by choosing among two achiral diketonate ligands, functioning as sensitizers (or antennae), combined with one of two chiral ligands to modulate the complex stereochemistry and therefore its CP emission.
Eu1 is formed by hexafluoroacetylacetonate (HFA) and 2,6-bis(4-isopropyl-2-oxazolin-2-yl)pyridine (iPrPyBox),17 while Eu2 and Eu3 feature thenoyltrifluoroacetone (TTA) and iPrPyBox and 2,6-bis(4-phenyl-2-oxazolin-2-yl)pyridine (PhPyBox) respectively (Scheme 1).17,18 Both HFA and TTA diketonates are efficient sensitizers for Eu,19,20 and the modified tridentate pyridine bisoxazoline (PyBox) ligands have proven effective in inducing highly CP–PL in this type of complexes in solution. A full characterization of the compounds is reported elsewhere.17,18
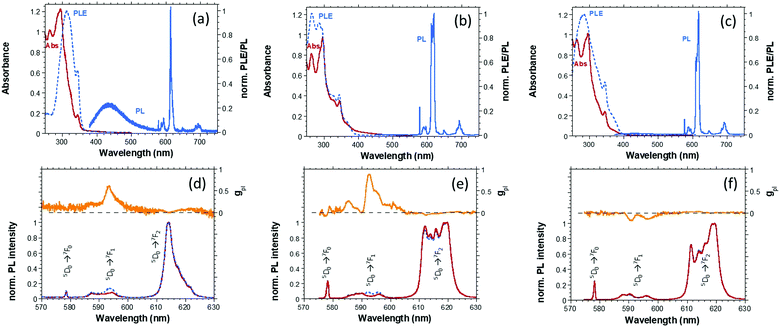
The optical properties of the three Eu(III) complexes (Fig. 1 and Table 1) are studied in toluene solutions and solid-state (thin film) samples by absorption, steady-state PL and time-resolved (TrPL) emission spectroscopy, PLQY, together with their CP–PL activity expressed as the PL dissymmetry factor gpl. Here only the properties of the S,S-enantiomer of the three complexes are discussed, while those of the corresponding R,R-enantiomer are reported in the ESI.† Then, the complexes are incorporated as intrinsic emitters in the active layer of solution processed OLED architectures.
| Compound | Solution | Film | Devicesd | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PMMA | PVK:OXD7 | ||||||||
| PLQYa | τ (μs)b | g pl (peak, nm) | PLQYc | τ (μs)b | g pl (peak, nm) | τ, (μs)b | g pl (Peak, nm) | g el (peak, nm) | |
| a λ ex = 365 nm. b λ ex = 320 nm, λem = 614 nm (Eu1); λex = 350 nm, λem = 619 nm (Eu2, Eu3). c Integrating sphere. d With transparent cathode. | |||||||||
| Eu1 | 0.30 | 868 | 0.30 (585) | 0.52 | 1029 | 0.30 (585) | 658 | 0.2 (585) | — |
| 0.76 (592) | 0.70 (593) | 0.6 (593) | |||||||
| −0.02 (614) | −0.02 (614) | — | |||||||
| Eu2 | 0.30 | 574 | 0.25 (585) | 0.55 | 689 | 0.21 (585) | 565 | 0.26 (585) | 0.16 (585) |
| 0.75 (592) | 0.59 (592) | 0.88 (592) | 0.51 (592) | ||||||
| −0.06 (612) | −0.03 (612) | −0.06 (612) | −0.04 (612) | ||||||
| Eu3 | 0.32 | 508 | −0.22 (592) | 0.62 | 667 | −0.18 (591) | 467 | −0.17 (591) | −0.22 (591) |
| −0.22 (596) | −0.17 (597) | −0.15 (596) | −0.25 (597) | ||||||
| 0.04 (614) | 0.03 (614) | 0.03 (614) | |||||||
Eu(III) complexes in solution exhibit a main absorption band, associated to the π–π* transition of the β-diketonate ligand, with a maximum at about 309 nm for Eu1 and 346 nm for Eu2 and Eu3 (Fig. S1a–c, ESI†). The absorption of PyBox ligands falls in the spectral region below 300 nm (not reported)18 and does not play a crucial role in the luminescence sensitization. The optical energy gaps (Eoptg) are derived from the onset of absorption spectra. All the Eu(III) complexes display good PLQYs of about 0.3, and relatively intense CP–PL activity which shows typical features of Eu(III) complexes (Fig. S1d–f, ESI†). We focus the analysis to the 580–630 nm range where the polarization of the transitions is more relevant. In fact, the strongest circular polarization occurs for the 5D0 → 7F1 magnetic transition in the range 585–595 nm, while the gpl is weaker for the 5D0 → 7F2 electronic transition in the range 610–620 nm with opposite sign with respect to the magnetic band. It is important to notice that CP brightness, and therefore the differential output of CP photons, is similar for the two bands in these cases, as often found for Eu complexes.21 Indeed, even for the weak 5D0 → 7F1 transition, such complexes display very large CP brightness factors21 (256, 243, 98 M−1 cm−1 for Eu1–3 respectively in toluene solution). Although the CP brightness as defined in ref. 21 does not directly apply in EL, where no light absorption is involved, the relative intensity of the various 5D0 → 7FX transitions is not significantly different in PL and EL and moreover the gpl and gel factors for the magnetic band are comparable (vide infra). This ensures that the differential flow of photons with opposite circular polarization emitted within the 5D0 → 7F1 transition remains very relevant even in CP–EL.
(S,S) Eu1 and (S,S) Eu2 show the largest gpl values of 0.77 and 0.75 at 592 nm, respectively, while (S,S) Eu3 features gPL peaks of −0.22 at 592 and 596 nm. Notably, an opposite sense of chirality can be obtained for the same absolute configuration of PyBox ligand bearing different side arms in Eu(III) TTA-based complexes.17,18,22 In fact, the sign of the CP–PL for (S,S) Eu1 and Eu2 (both bearing (S,S)-iPrPyBox) is positive for the 5D0 → 7F1 transition, and it is reversed for (S,S) Eu3. This confirms that the substituents on the chiral ancillary ligand affect the CP emission signature independently from the diketonate used. The photophysics of Eu(III) complexes dispersed in host matrices are evaluated as thin films in view of their use in the active layer of CP-OLEDs. Firstly, the dispersion of Eu1–3 in a poly(methyl methacrylate) (PMMA) matrix offers the opportunity to study Eu(III) complexes behaviour in rigid and neutral environment.
Then the dispersion of Eu1–3 in the active matrix used for OLEDs is investigated. Poly(9-vinylcarbazole) (PVK) together with 1,3-bis[2-(4-tert-butylphenyl)-1,3,4-oxadiazo-5-yl]benzene (OXD7) is the most widely used bipolar host already successfully employed with Ln(III) complexes.13 The Eu(III) complexes are dispersed in PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OXD7 (whose ratio is set to 3
OXD7 (whose ratio is set to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%.) with a fixed concentration of 8.5% by mass with respect to the matrix.
1 wt%.) with a fixed concentration of 8.5% by mass with respect to the matrix.
Absorption spectra of the PMMA blends (Fig. S2a–c, ESI†) resemble the solutions ones, while spectra of PVK:OXD7:Eu1–3 films (Fig. 1a–c) are dominated by the PVK:OXD7 contribution.
Importantly, the CP activity is retained in the blends (Fig. 1). The ECD spectra of PVK:OXD7:Eu1–3 films (Fig. S3–S5, ESI†), despite a weak signal due to the low concentration of the complexes in the polymer blend, is similar to the spectra obtained in solution.17,18 This indicates that a similar geometry of the complexes is preserved in the active-matrix blend. In particular, in the ECD spectrum of (R,R) Eu3 blend, the main Cotton effect (around 360 nm, associated with TTA π→ π* transition) is inverted with respect to the main band observed in the case of (R,R) Eu1 and (R,R) Eu2 (315 and 340 nm respectively). On the other hand, the spectra of (R,R) Eu1 and (R,R) Eu2 blends are comparable, except for the blue shift of Eu1, due to a higher HOMO–LUMO gap in the HFA vs. TTA. These data point towards a similar arrangement of the ligands around the metal centre for Eu1 and Eu2 complexes due to the presence of iPrPyBox, while an almost mirror image coordination geometry of the TTA ligands is at play where the chirality induction is led by PhPyBox, as in the case of Eu3. Moreover, no induced ECD onto the polymer blend is observed, excluding any significant chirality transfer from the complex to the matrix, as it is instead the case with, e.g., some helicene/achiral polymer systems.23
The values of gpl recorded for Eu1 complex dispersed in the blends are slightly lower than in the corresponding toluene solutions (Table 1). (S,S) Eu1 features similar gpl of 0.7 and 0.6 at 593 nm in PMMA and PVK:OXD7 blends, respectively, while (S,S) Eu2 shows the highest value once dispersed in PVK:OXD7 (0.88 with respect to 0.59 in PMMA matrix). (S,S) Eu3 displays lower (and again reverse in sign) gpl with respect to (S,S) Eu1–2 at two different wavelengths (−0.17 and −0.15 at 591 and 597 nm in PVK:OXD7 blend).
PL excitation spectrum (PLE) of PVK:OXD7:Eu1 film (Fig. 1a), recorded by monitoring the emission at 614 nm, is very similar to the absorption of Eu1 solution (Fig. S1a, ESI†) mainly associated to HFA transitions, and does not follow the absorption of the film (except for a weak PVK band at 344 nm), meaning that negligible Förster resonant energy transfer (FRET) from host (excitation donor) to Eu1 (acceptor) takes place. Accordingly, the broad PVK emission at about 440 nm is observed in the blend (Fig. 1a) once excited at 310–350 nm. On the contrary, the PLE spectra of both Eu2 and Eu3 blend films (Fig. 1b and c) resemble their absorption spectra (Fig. S1b and c, ESI†) indicating that efficient FRET occurs from the host matrix, whose emission is completely quenched (Fig. 1b and c). The observed different efficiency of FRET from PVK to the Eu(III) complexes is consequence of the lack of spectral overlap between Eu1 absorption and PVK emission, while a good overlap is observed for Eu2 or Eu3 (see Fig. S6, ESI†). Generally, the FRET mechanism promotes the radiative recombination at acceptor sites, hence positively impacting on device performance. However, excitation back transfer from Eu(III) complex ligand to the host triplet energy level represents a major quenching mechanism in PVL:OXD7:Eu(III) complexes films.24 This is more critical for Eu1, whose triplet energy (2.65 eV) is higher than the PVK one (2.5 eV, Fig. S8, ESI†).
TrPLs of the Eu1–3, both in solution and blends, were performed (Fig. S7, ESI†) to support this scenario. All the complexes display longer lifetimes in the rigid PMMA matrix with respect to the solution, with Eu1 displaying the longest lifetimes among the three complexes (Table 1). The decays are systematically faster in PVK:OXD7 than in PMMA blends. This is particularly evident for complex Eu1, whose lifetime and PLQY (0.24) in the PVK:OXD7 blend decreases by about a factor 2 when compared to the PMMA blend.
This analysis indicates that the diketonate ligand (TTA vs. HFA) plays a main role in both the exciton recombination process and in ET from the polymer host. In particular, among the three complexes, the use of Eu1 in solution processed OLEDs with PVK-based active layers appears unfavourable both for the emission quenching induced by back-transfer to PVK and for the absence of FRET processes.
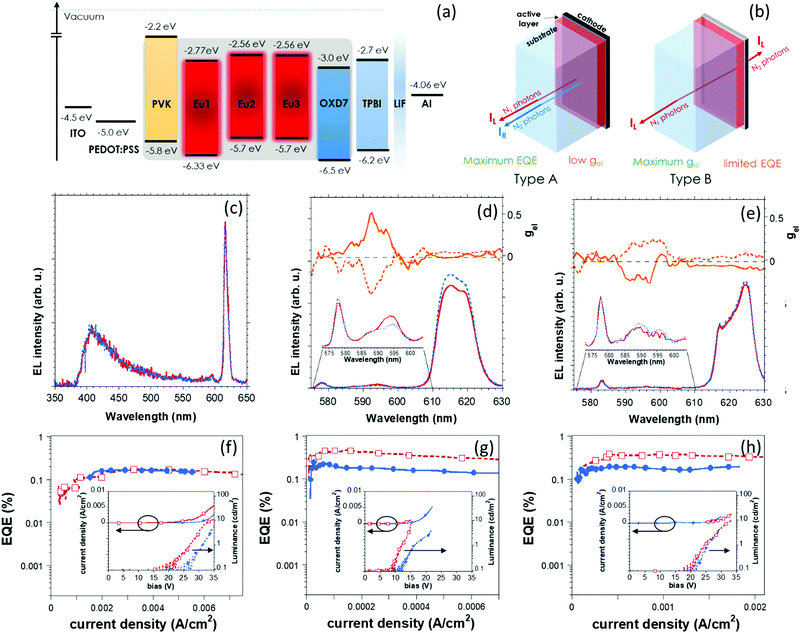
Cyclic voltammetry of Eu(III) complexes was performed to obtain molecular energy levels of the complexes from the measurement of their oxidation potentials (Fig. S9, ESI†) and optical gap. The determined LUMO value is −2.77 for Eu1 and −2.56 eV for Eu2 and Eu3. The HOMO level is −6.33 and −5.77 eV for Eu1 and Eu2–Eu3 respectively (Fig. 2a).
The devices were then fabricated with the architecture ITO/PEDOT:PSS/PVK:OXD7:Eu(III) complexes/TPBI/Ba/Al [TPBI: 2,2′,2′′-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole)] whose flat band energy level diagram is reported in Fig. 2a.
The active layer (80 nm thick) is processed by spin-coating from 15 mg ml−1 CHCl3 degassed solution over PEDOT:PSS-covered ITO/glass substrate. The formulation of the blend is the same used for the photophysical characterization (the content of Eu(III) complex is fixed to 8.5 wt.% with respect to the host matrix, see Fig. S7, ESI†) as it ensures both superior performance of the device and pure Eu-centred EL. A 30 nm thick TPBI electron transporting/hole blocking layer, thermo-sublimated in high vacuum on top of the active layer, is finally covered by the LiF/Al cathode deposition.
Devices are fabricated with two different strategies to maximize the performance in term of external quantum efficiency (EQE, measured in forward direction) (hereafter called devices of type A) or CP–EL (type B, see Fig. 2b). In fact, since the transparency of the cathode mostly impacts on the EQE of the devices,12,13,25 type A devices are designed with a reflective cathode of 1.5 nm of LiF capped by 110 nm of aluminium to allow for a maximal collection of the photons in forward direction (maximum EQE). On the other hand, reflection on the back electrode causes a reversal of handedness of CP light and therefore a decrease of polarization performances. Thus, in devices of type B, the thickness of aluminium capping is reduced to 6 nm (while LiF remained unchanged) to be almost transparent to red light and to provide higher EL dissymmetry factors. All the devices exhibit EL spectra with typical features of Eu(III) emission. As expected, the higher EQE, measured in forward direction, is observed in type A devices (Fig. S10, ESI†).12 EQE of Eu2-based devices reaches the value of 0.48%, which is the highest reported for chiral Europium-based OLEDs and exceeds of one order of magnitude the earlier reported results.13 In corresponding type B devices, the EQE is slightly lower (0.22%), but a remarkable EL dissymmetry factor gel of ∼0.51 [for (S,S) enantiomer] indicates that at 592 nm (Fig. 2d), a high degree of CP photons (63% of them are left polarized) can be achieved in EL as well, once the reflection on the back electrode is reduced (Table 1). Type A devices based on Eu1 and Eu3 showed EQE of 0.17% and 0.38% respectively. These figures are roughly in the range of the ones reported for regular (i.e. non-polarized) OLEDs based on Eu complexes.26 As expected from CP–PL spectra, with the same absolute configuration of the PyBox ligand, Eu3-based devices show opposite sign gel with respect to Eu2-based ones (namely gel +0.51/−0.25 for 5D0 → 7F1 transition in the case of (S,S) Eu2 and Eu3 respectively, Fig. 2d, e and Table 1). CP–EL spectra obtained for devices containing enantiomer complexes are mirror image, ensuring the reliability of the polarization data measured for the devices.
It must be noted that good PLQYs measured for Eu1–3 does not guarantee good emission performance in a device. In fact, the low HOMO level of Eu1 with respect to Eu2 and Eu3 most probably prevents direct charge trapping upon biasing, and, together with the observed lack of FRET, promotes a contribution of the PVK blue emission to the EL spectrum (see Fig. 2c), affecting the performance of the device. Moreover, the high driving voltages of Eu1-based devices (20–25 V, Fig. S11, ESI†), due to larger hole injection energy barriers of Eu1 with respect to Eu2–3 (Fig. 2a), causes unstable emission that prevents reliable determination of gel. Further, the significant reduction of PL decay time observed in Eu1,3 blended in PVK:OXD7 matrix, compared to PMMA, suggests that PVK:OXD7 is energetically not enough well-suited27 to dilute Eu1,3 with respect to Eu2. All devices show low roll-off efficiency, but a modest luminance (10 cd m−2 at about 15 V for Eu2 and 30–35 V for Eu1,3-based devices), leaving room for an improvement by exploring different host matrices.14,26
Conclusions
In conclusion, we obtained CP-OLED devices with peculiar architectures based on Ln(III) complexes showing both remarkable emission efficiency (EQE = 0.48%) and polarization performances (∣gel∣ = 0.51). This was achieved by taking advantage of a modular strategy for the design of the Ln(III) emitter. Lanthanide chemistry allows for the easy preparation of heteroleptic complexes, that is of complexes containing two different ligands. In the first place, three diketonates are used to sensitize Ln(III) emission, while providing stable coordination and a neutral core. Although from a spectroscopic point of view TTA and HFA may be considered similar, the ability of the former to harvest exciton recombination events in the OLED architecture developed herein is far superior, compared to the latter. Secondly, a chiral ligand with nitrogen donors completes the Ln(III) coordination sphere, endowing the complex with the necessary dissymmetry and controlling the circular polarization of the device. We believe that such modular strategy can be effective to adapt lanthanide complexes to the multiple requirements of efficient CP-OLEDs.Author contributions
FZ and LDB conceived the idea, FZ and LA prepared the complexes and performed preliminary (chiro)optical characterization, UG manufactured CP-OLEDs and carried out device characterization. MP optimized the formulation of the blends and performed film deposition. CB performed the photophysical characterization. TF performed electrochemical analysis. FZ, UG wrote the manuscript, all the authors read and approved the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Italian University and Research Ministry (PRIN Project 20172M3K5N) is gratefully acknowledged. FZ and LDB thank the University of Pisa for financial support (PRA 2020_21 and 2019_23).References
- Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light: Sci. Appl., 2021, 10, 76 CrossRef CAS PubMed.
- H. Kuang, C. Xu and Z. Tang, Adv. Mater., 2020, 32, 2005110 CrossRef CAS PubMed.
- G. Yang, M. Kazes and D. Oron, Adv. Funct. Mater., 2018, 28, 1802012 CrossRef.
- S. Ma, J. Ahn and J. Moon, Adv. Mater., 2021, 2005760 CrossRef CAS PubMed.
- T. Mori, “Circularly Polarized Luminescence of Isolated Small Organic Molecules”, Springer, 2020 Search PubMed.
- J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 0045 CrossRef CAS.
- R. Farshchi, M. Ramsteiner, J. Herfort, A. Tahraoui and H. T. Grahn, Appl. Phys. Lett., 2011, 98, 162508 CrossRef.
- J. M. Han, S. Guo, H. Lu, S. J. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 RSC and reference therein.
- X. Zhang, Y. Zhang, Y. Li, Y. Quan, Y. Cheng and Y. Li, Chem. Commun., 2019, 55, 9845 RSC; Z.-G. Wu, H.-B. Han, Z.-P. Yan, X.-F. Luo, Y. Wang, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Adv. Mater., 2019, 31, 1900524 CrossRef CAS PubMed; M. Li, Y.-F. Wang, D. Zhang, L. Duan and C.-F. Chen, Angew. Chem., Int. Ed., 2020, 59, 3500 CrossRef PubMed; L. Frédéric, A. Desmarchelier, R. Plais, L. Lavnevich, G. Muller, C. Villafuerte, G. Clavier, E. Quesnel, B. Racine, S. Meunier-Della-Gatta, J.-P. Dognon, P. Thuéry, J. Crassous, L. Favereau and G. Pieters, Adv. Funct. Mater., 2020, 30, 2004838 CrossRef PubMed; Y.-P. Zhang, X. Liang, X.-F. Luo, S.-Q. Song, S. Li, Y. Wang, Z.-P. Mao, W.-Y. Xu, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Angew. Chem., Int. Ed., 2021, 60, 8435 CrossRef PubMed; K. Dhbaibi, L. Abella, S. Meunier-Della-Gatta, T. Roisnel, N. Vanthuyne, B. Jamoussi, G. Pieters, B. Racine, E. Quesnel, J. Autschbach, J. Crassous and L. Favereau, Chem. Sci., 2021, 12, 5522–5533 RSC; M. Li, M.-Y. Wang, Y.-F. Wang, L. Feng and C.-F. Chen, Angew. Chem., Int. Ed., 2021, 60, 20728 CrossRef PubMed.
- T. Y. Li, Y. M. Jing, X. Liu, Y. Zhao, Y.-X. Zheng and J.-L. Zuo, Sci. Rep., 2015, 5, 14912 CrossRef CAS PubMed; J. M. Han, S. Guo, J. Wang, L. W. Wei, Y. L. Zhuang, S. J. Liu, Q. Zhao, X. W. Zhang and W. Huang, Adv. Opt. Mater., 2017, 5, 1700359 CrossRef; Z.-P. Yan, X.-F. Luo, W.-Q. Liu, Z.-G. Wu, X. Liang, K. Liao, Y. Wang, Y.-X. Zheng, L. Zhou, J.-L. Zuo, Y. Pan and H. Zhang, Chem. – Eur. J., 2019, 25, 5672 CrossRef PubMed; Z.-P. Yan, K. Liao, H.-B. Han, J. Su, Y.-X. Zheng and J.-L. Zuo, Chem. Commun., 2019, 55, 8215 RSC; Y. Chen, X. Li, N. Li, Y. Quan, Y. Cheng and Y. Tang, Mater. Chem. Front., 2019, 3, 867 RSC; G. Lu, Z.-G. Wu, R. Wu, X. Cao, L. Zhou, Y.-X. Zheng and C. Yang, Adv. Funct. Mater., 2021, 31, 2102898 CrossRef.
- F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791 CrossRef CAS PubMed.
- F. Zinna, M. Pasini, F. Galeotti, C. Botta, L. Di Bari and U. Giovanella, Adv. Funct. Mater., 2017, 27, 1603719 CrossRef.
- Y. Yang, R. C. da Costa, D.-M. Smilgies, A. J. Campbell and M. J. Fuchter, Adv. Mater., 2013, 25, 2624 CrossRef CAS PubMed; J. R. Brandt, X. Wang, Y. Yang, A. J. Campbell and M. J. Fuchter, J. Am. Chem. Soc., 2016, 138(31), 9743 CrossRef PubMed; D.-M. Lee, J.-W. Song, Y.-J. Lee, C.-J. Yu and J.-H. Kim, Adv. Mater., 2017, 29, 1700907 CrossRef PubMed; D. Di Nuzzo, C. Kulkarni, B. Zhao, E. Smolinsky, F. Tassinari, S. C. J. Meskers, R. Naaman, E. W. Meijer and R. H. Friend, ACS Nano, 2017, 11(12), 12713 CrossRef PubMed; F. Song, Z. Xu, Q. Zhang, Z. Zhao, H. Zhang, W. Zhao, Z. Qiu, C. Qi, H. Zhang, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam, Z. Zhao, A. Qin, D. Ma and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1800051 CrossRef.
- F. Zinna and L. Di Bari, Chirality, 2015, 27, 1 CrossRef CAS PubMed; H.-Y. Wong, W.-S. Lo, K.-H. Yim and G.-L. Law, Chem, 2019, 5(12), 3058 Search PubMed; L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2021, 5, 109 CrossRef.
- H. Xu, Q. Sun, Z. An, Y. Wei and X. Liu, Coord. Chem. Rev., 2015, 293–294, 228 CrossRef CAS.
- J. Yuasa, T. Ohno, K. Miyata, H. Tsumatori, Y. Hasegawa and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9892 CrossRef CAS PubMed.
- M. Górecki, L. Carpita, L. Arrico, F. Zinna and L. Di Bari, Dalton Trans., 2018, 47, 7166 RSC.
- J.-C. G. Bünzli and S. V. Eliseeva, in Basics of Lanthanide Photophysics. Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects, ed. P. Hänninen and H. Härmä, Springer, Heidelberg, 2010, p. 1 Search PubMed.
- C. Freund, W. Porzio, U. Giovanella, F. Vignali, M. Pasini, S. Destri, A. Mech, S. Di Pietro, L. Di Bari and P. Mineo, Inorg. Chem., 2011, 50, 5417 CrossRef CAS PubMed.
- L. Arrico, L. Di Bari and F. Zinna, Chem. – Eur. J., 2021, 27, 2920 CrossRef CAS PubMed; Y. Nagata and T. Mori, Front. Chem., 2020, 8, 448 CrossRef PubMed.
- F. Zinna, L. Arrico and L. Di Bari, Chem. Commun., 2019, 55, 6607 RSC.
- J. Wade, J. R. Brandt, D. Reger, F. Zinna, K. Y. Amsharov, N. Jux, D. L. Andrews and M. J. Fuchter, Angew. Chem., Int. Ed., 2021, 60, 222 CrossRef CAS PubMed.
- U. Giovanella, M. Pasini, C. Freund, C. Botta, W. Porzio and S. Destri, J. Phys. Chem. C, 2009, 113(6), 2290 CrossRef CAS.
- F. Zinna, G. Pescitelli and L. Di Bari, Chirality, 2020, 32, 765 CrossRef CAS PubMed.
- L. Wang, Z. Zhao, C. Wei, H. Wei, Z. Liu, Z. Bian and C. Huang, Adv. Opt. Mater., 2019, 7, 1801256 CrossRef.
- T. W. Canzler and J. Kido, Org. Electron., 2006, 7, 29 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tc05023k |
| This journal is © The Royal Society of Chemistry 2022 |