Indeno-anthraquinone hosts with thermally activated delayed fluorescence for deep-red OLEDs†
Abstract
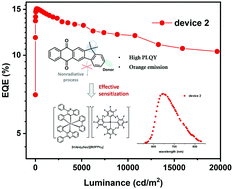
High-efficiency materials exhibiting thermally activated delayed fluorescence (TADF) are highly promising for organic light-emitting diodes (OLEDs). A rational acceptor core based on indeno-anthraquinone (IAQ) was proposed in this work. It showed molecular ridigity and expanded conjugation for construction of orange–red TADF. Two TADF molecules involving IAQ were designed and synthesized. They simultaneously possessed high photoluminescence quantum efficiency (PLQY) and efficient reverse intersystem crossing (RISC) for red cationic iridium phosphor to construct TADF-sensitized phosphorescent (TSP) OLEDs. As a result, a stable OLED device exhibiting deep-red emission was fabricated with a maximum external quantum efficiency (EQEmax) of 15.1% and Commission Internationale de L’Eclairage coordinate of (0.69, 0.31).
- This article is part of the themed collection: Materials for thermally activated delayed fluorescence and/or triplet fusion upconversion


 Please wait while we load your content...
Please wait while we load your content...