Nanomaterial integrated 3D printing for biomedical applications
Abstract
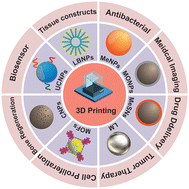
3D printing technology, otherwise known as additive manufacturing, has provided a promising tool for manufacturing customized biomaterials for tissue engineering and regenerative medicine applications. A vast variety of biomaterials including metals, ceramics, polymers, and composites are currently being used as base materials in 3D printing. In recent years, nanomaterials have been incorporated into 3D printing polymers to fabricate innovative, versatile, multifunctional hybrid materials that can be used in many different applications within the biomedical field. This review focuses on recent advances in novel hybrid biomaterials composed of nanomaterials and 3D printing technologies for biomedical applications. Various nanomaterials including metal-based nanomaterials, metal–organic frameworks, upconversion nanoparticles, and lipid-based nanoparticles used for 3D printing are presented, with a summary of the mechanisms, functional properties, advantages, disadvantages, and applications in biomedical 3D printing. To finish, this review offers a perspective and discusses the challenges facing the further development of nanomaterials in biomedical 3D printing.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...