Membrane-fusogenic biomimetic particles: a new bioengineering tool learned from nature
Abstract
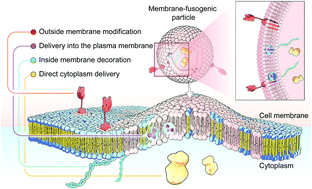
Membrane fusion, a fundamental biological process of the fusion of the membrane composition between cells, is vital for cell–cell communication and cargo transport between living cells. This fusion interaction achieves the transportation of the inner content to the cellular cytosol as well as the simultaneous blending of foreign substances with the cell membrane. Inspired by this biological process, emerging membrane-fusogenic particles have been developed, opening a new area for bioengineering and biomedical applications. Especially, membrane-fusion-mediated transfer of inner cargoes can bypass endosomal entrapment to maximize the transportation efficiency, emerging as a unique cytoplasmic delivery platform distinct from those depending on conventional endocytosis-based pathways. In addition, the membrane fusion enables cell surface modification through lipid diffusion and mixing, providing a tool for direct cell membrane engineering. In this review, we focus on the development of membrane-fusogenic particles and their up-to-date progress. We briefly introduce the concept of membrane fusion, elaborate inspiring strategies of membrane-fusogenic particles, and highlight the recent advances and the promising applications of membrane-fusogenic particles as a next-generation bioengineering tool. In the end, we conclude with the present challenges and opportunities, providing insights in the future research of membrane-fusogenic particles.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...