Latest on biomaterial-based therapies for topical treatment of psoriasis
Abstract
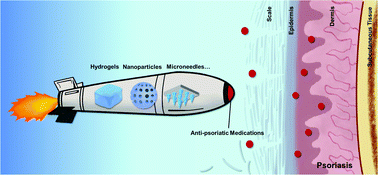
Psoriasis is an autoimmune inflammatory disease which is fundamentally different from dermatitis. Its treatments include topical medications and systemic drugs depending on different stages of the disease. However, these commonly used therapies are falling far short of clinical needs due to various drawbacks. More precise therapeutic strategies with minimized side effects and improved compliance are highly demanded. Recently, the rapid development of biomaterial-based therapies has made it possible and promising to attain topical psoriasis treatment. In this review, we briefly describe the significance and challenges of the topical treatment of psoriasis and emphatically overview the latest progress in novel biomaterial-based topical therapies for psoriasis including microneedles, nanoparticles, nanofibers, and hydrogels. Current clinical trials related to each biomaterial are also summarized and discussed.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...