Emerging nanobiotechnology-encoded relaxation tuning establishes new MRI modes to localize, monitor and predict diseases
Abstract
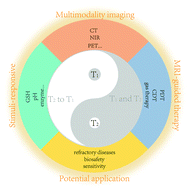
Magnetic resonance imaging (MRI) is one of the most important techniques in the diagnosis of many diseases including cancers, where contrast agents (CAs) are usually necessary to improve its precision and sensitivity. Previous MRI CAs are confined to the signal-to-noise ratio (SNR) elevation of lesions for precisely localizing lesions. As nanobiotechnology advances, some new MRI CAs or nanobiotechnology-enabled MRI modes have been established to vary the longitudinal or transverse relaxation of CAs, which are harnessed to detect lesion targets, monitor disease evolution, predict or evaluate curative effect, etc. These distinct cases provide unexpected insights into the correlation of the design principles of these nanobiotechnologies and corresponding MRI CAs with their potential applications. In this review, first, we briefly present the principles, classifications and applications of conventional MRI CAs, and then elucidate the recent advances in relaxation tuning via the development of various nanobiotechnologies with emphasis on the design strategies of nanobiotechnology and the corresponding MRI CAs to target the tumor microenvironment (TME) and biological targets or activities in tumors or other diseases. In addition, we exemplified the advantages of these strategies in disease theranostics and explored their potential application fields. Finally, we analyzed the present limitations, potential solutions and future development direction of MRI after its combination with nanobiotechnology.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...