Bioactive hydrogels based on polysaccharides and peptides for soft tissue wound management
Abstract
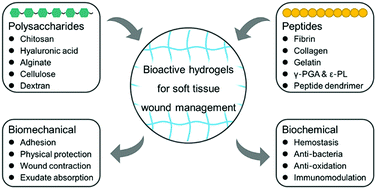
Due to their inherent and tunable biomechanical and biochemical performances, bioactive hydrogels based on polysaccharides and peptides have shown attractive potential for wound management. In this review, the recent progress of bioactive hydrogels prepared by polysaccharides and peptides for soft tissue wound management is overviewed. Meanwhile, we focus on the elaboration of the relationship between chemical structures and inherent bioactive functions of polysaccharides and peptides, as well as the strategies that are taken for achieving multiple wound repairing effects including hemostasis, adhesion, wound contraction and closure, anti-bacteria, anti-oxidation, immunomodulation, molecule delivery, etc. Some innovative and important works are well introduced as well. In the end, current study limitations, clinical unmet needs, and future directions are discussed.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...