Red to NIR-emissive anthracene-conjugated PMI dyes with dual functions: singlet-oxygen response and lipid-droplet imaging†
Abstract
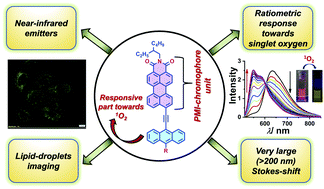
The rich chemistry of solution-processable red and near-infrared (NIR) organic emitters has emerged as an attractive and progressive research field because of their particular applications in organic optoelectronics and bioimaging. Also, one can see that the research area of perylene monoimide-based red and NIR-emissive fluorophores is underexplored, which prompted us to design and synthesize three anthracene-conjugated PMI dyes exhibiting strong emission in the red and NIR window in solution. Three PMI-based fluorophores were synthesized via conjoining anthracene and donor moieties (-Ph, -N,N-PhNMe2) with a PMI core via an acetylene linkage at the peri-position, which helped to attain extensive electronic conjugation, which was reflected in red and NIR-emission in solution. The key molecular features to be highlighted here are: all three dyes are strongly emissive in solution, as unveiled by the excellent absolute fluorescence QYs; and they possess tuneable emission properties, guided by the donor strength and a profound Stokes shift (100–200 nm). The three fluorescent dyes demonstrated appreciable singlet-oxygen (1O2) sensitivity when photoirradiated with methylene blue (MB) in solution, showing a substantial blue-shift in emission in a ratiometric manner. Further, the treatment of dye–MB solution with α-tocopherol (1O2 scavenger) validated the presence of 1O2 as the only oxidizing species generated by MB in solution. Computational investigations gave insight into the twisting of donor moieties in their ground-state optimized geometries, the modulation of the FMO energy gap, and the thermodynamic feasibility of the 1O2 reaction. Finally, via taking advantage of the red and NIR-emission, we successfully utilized one of the fluorophores as a lipid-droplet marker for bioimaging in HepG2 cells.


 Please wait while we load your content...
Please wait while we load your content...