Molybdenum disulfide (MoS2)-based nanostructures for tissue engineering applications: prospects and challenges
Abstract
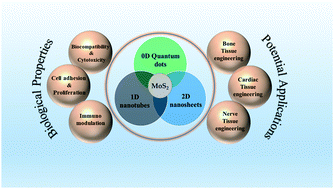
Molybdenum disulfide (MoS2) nanostructures have recently earned substantial thoughts from the scientific communities owing to their unique physicochemical, optical and electrical properties. Although MoS2 has been mostly highlighted for its industrial applications, its biological applicability has not been extensively explored. The introduction of nanotechnology in the field of tissue engineering has significantly contributed to human welfare by displaying advancement in tissue regeneration. Assimilation of MoS2 nanostructures into the polymer matrix has been considered a persuasive material of choice for futuristic tissue engineering applications. The current review provides a general discussion on the structural properties of different MoS2 nanostructures. Further, this article focuses on the interactions of MoS2 with biological systems in terms of its cellular toxicity, and biocompatibility along with its capability for cell proliferation, adhesion, and immunomodulation. The article continues to confer the utility of MoS2 nanostructure-based scaffolds for various tissue engineering applications. The article also highlights some emerging prospects and possibilities of the applicability of MoS2-based nanostructures in large organ tissue engineering. Finally, the article concludes with a brief annotation on the challenges and limitations that need to be overcome in order to make plentiful use of this wonderful material for tissue engineering applications.
- This article is part of the themed collection: 2023 Journal of Materials Chemistry B Lunar New Year


 Please wait while we load your content...
Please wait while we load your content...