Self-targeting of zwitterion-based platforms for nano-antimicrobials and nanocarriers
Abstract
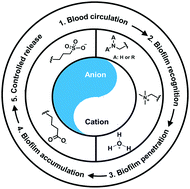
Self-targeting antimicrobial platforms have yielded new possibilities for the treatment of infectious biofilms. Self-targeting involves stealth transport through the blood circulation towards an infectious biofilm, where the antimicrobial platform penetrates and accumulates in a biofilm in response to a change in environmental conditions, such as local pH. In a final step, nano-antimicrobials need to be activated or the antimicrobial cargo of nanocarriers released. Zwitterions possess both cationic and anionic groups, allowing full reversal in zeta potential from below to above zero in response to a change in environmental conditions. Electrolyte-based platforms generally do not have the ability to change their zeta potentials from below to above zero. Zwitterions for use in self-targeting platforms are usually hydrophilic and have a negative charge under physiological conditions (pH 7.4) providing low adsorption of proteins and assisting blood circulation. However, near or in the acidic environment of a biofilm, they become positively-charged yielding targeting, penetration and accumulation in the biofilm through electrostatic double-layer attraction to negatively-charged bacteria. Response-times to pH changes vary, depending on the way the zwitterion or electrolyte is built in a platform. Self-targeting zwitterion-based platforms with a short response-time in vitro yield different accumulation kinetics in abdominal biofilms in living mice than platforms with a longer response-time. In vivo experiments in mice also proved that self-targeting, pH-responsive zwitterion-based platforms provide a feasible approach for clinical control of bacterial infections. Clinically however, also other conditions than infection may yield an acidic environment. Therefore, it remains to be seen whether pH is a sufficiently unique recognition sign to direct self-targeting platforms to an infectious biofilm or whether (additional) external targeting through e.g. near-infrared irradiation or magnetic field application is needed.
- This article is part of the themed collection: Bioinspired Surfaces Engineering for Biomaterials


 Please wait while we load your content...
Please wait while we load your content...