Preclinical testing of an anal bulking agent coated with a zwitterionic polymer in a fecal incontinence rat model†
Jung-Woo
Choi‡
a,
Joonbum
Lee‡
b,
Yuseon
Lee
c,
Ji-Hun
Seo
 *b and
Kwang Dae
Hong
*d
*b and
Kwang Dae
Hong
*d
aDepartment of Pathology, Korea University Ansan Hospital, Gyeonggi-do, Republic of Korea
bDepartment of Materials Science and Engineering, Korea University, Seoul, Republic of Korea. E-mail: seojh79@korea.ac.kr
cBiomedical Research Center, Korea University Ansan Hospital, Gyeonggi-do, Republic of Korea
dDepartment of Colorectal Surgery, Korea University Ansan Hospital, Gyeonggi-do, Republic of Korea. E-mail: drhkd@korea.ac.kr
First published on 3rd February 2022
Abstract
Fecal incontinence is a disabling condition in which the passage of fecal material cannot be controlled. Although the condition is not life-threatening, it can seriously reduce the quality of life of a patient by isolating them from others. Though various surgical treatments are available for moderate to severe symptoms, a bulking agent is a minimally invasive technique that has attracted attention because of its safety and simple treatment process. However, the biocompatibility of bulking agent materials remains a central issue, with their durability questioned because immune responses and/or the circulatory system may remove the bulking agent in vivo. This study investigated a bulking agent composed of polydimethylsiloxane and hyaluronic acid as a microfiller and carrier gel, respectively. To improve the injectability of the bulking agent, the filler size was tuned using a suspension-based fabrication technique. To evade immune responses, the filler surface was treated with a zwitterionic polymer that simultaneously functionalized and stabilized the material interfaces. The resulting bulking agent exhibited good injectability and biocompatibility in vitro, with 58% lower protein adsorption and no cytotoxicity, leading to an improved bulking effect in a preclinical rat model compared with a bulking agent without surface treatment. These results illustrate the promising potential of bulking agents as a therapy for fecal incontinence with reduced foreign body reactions and long-lasting efficacy.
Introduction
Fecal incontinence (FI) is the involuntary loss of bowel content, including gas, liquid stool and mucus, and/or solid feces. The symptoms are generally a source of severe stress for the patient and can lead to social isolation. It has been estimated that FI affects up to 18% of the population,1 with childbirth trauma and abnormalities in the anal sphincter complex being the most common causes.2 When conservative treatments such as antidiarrheal agents and biofeedback fail, surgical intervention is required. The injection of a bulking agent around the anal sphincter muscle can augment the anal cushion and generate a passive barrier to the involuntary passage of a stool. A recent systematic review has found that this procedure exhibits good mid-term outcomes and minimal complications,3 but the optimal injection material has yet to be determined.Various materials have been tested for use in bulking agents, including collagen, carbon particles, silicone, dextranomer, and hydroxyapatite. However, none of these satisfy all of the characteristics of an ideal implant: biocompatibility, safety, stability at the implantation site, cost-effectiveness, and no migration.4 The implantation of a biomaterial inevitably induces the host immune response known as the foreign body reaction (FBR), which consists of a series of inflammatory reactions followed by fibrosis.5 Nonspecific protein adsorption onto the implant surface is considered the first step in the FBR. The interaction between the adsorbed proteins and the adhesion receptors on the inflammatory cells represents a major cellular recognition system for implanted biomaterials. Thus, the surface properties of the biomaterial are an important factor in modulating the FBR in the first 2–4 weeks following the implantation of the biomaterial, even though the FBR at the interface between the tissue and material will continue for the in vivo lifetime of the implant.6
Zwitterions are superhydrophilic materials with both cationic and anionic groups, and they can interact electrostatically with water molecules to form a hydration layer and inhibit nonspecific protein adsorption.7 Zwitterionic polymers such as 2-methacryloyloxyethyl phosphorylcholine (MPC), sulfobetaine methacrylate (SBMA), and carboxybetaine methacrylate (CBMA) have shown promise in various medical applications due to their anti-biofouling effects.8–11 Polydimethylsiloxane (PDMS), which is commonly referred to as silicone, has been used in various medical applications due to its versatile, inexpensive, and nontoxic properties.12–14 Hence, we hypothesized that if zwitterions are coated onto the surface of a PDMS elastomer, the resulting novel bulking agent could exhibit higher biocompatibility and cost-effectiveness.
In this work, we describe the formation of an injectable PDMS bulking agent and a durable zwitterionic coating thereon to reduce protein adsorption and cellular responses and thus mitigate the FBR. To this end, a hydrogel of hyaluronic acid, a major natural mucopolysaccharide in the body's synovial tissues and one of the most biocompatible materials, was used as an injection carrier. Hyaluronic acid and/or its hydrogels are particularly advantageous for injection purposes because of the lubricating and shear thinning properties.39,40 Using the proposed biomaterial, we investigated its functional and histological performance in an FI rat model (Scheme 1).
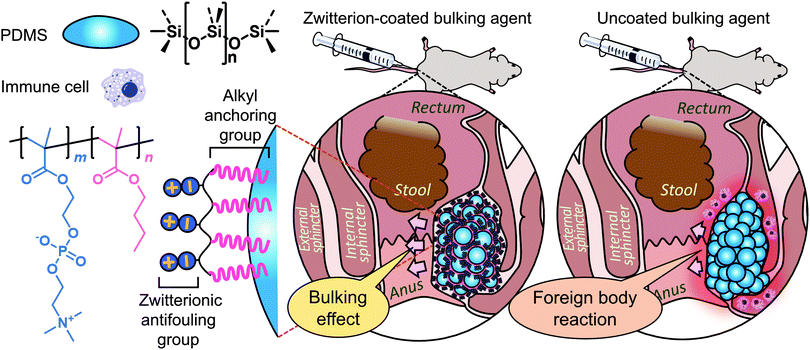 | ||
| Scheme 1 Schematic representation of the fabrication of zwitterion-coated bulking agent and its preclinical testing. PDMS, polydimethylsiloxane. | ||
Experimental
Materials
MPC was purchased from KCI, while n-butyl methacrylate (BMA) and 1,4-butanediol diglycidyl ether (BDDE) were purchased from Tokyo Chemical Industry. Anhydrous ethanol, diethyl ether, and sodium hydroxide solution (1 M) were purchased from Samchun Chemical. 2,2′-Azobisisobutyronitrile (AIBN) solution (0.2 M in toluene), polyvinylpyrrolidone (PVP), fluorescein isothiocyanate-labeled bovine serum albumin (FITC-BSA), and sodium nitrate was purchased from Sigma-Aldrich. PDMS (Sylgard 184 silicone elastomer) was purchased from Dow. Hyaluronic acid (HA) sodium salt (91%, molecular weight ≥ 1 MDa, from Streptococcus equi) was purchased from Alfa Aesar. Deionized water was prepared using an ultrapure water system (Milli-Q IQ 7000) from Merck Millipore. A regenerated cellulose dialysis membrane (SpectraPor; molecular weight cut-off, 12–14 kDa) was purchased from Repligen. For the cell cultures, an embryotic mouse fibroblast cell line (NIH/3T3) was taken from the Korean Collection for Type Cultures (KCTC). Phosphate-buffered saline (PBS) was purchased from Welgene and other bioreagents, including Dulbecco's phosphate-buffered saline, trypsin-EDTA solution (0.25%), Dulbecco's modified Eagle's medium (DMEM), and newborn calf serum (NBCS), were purchased from Gibco. A water-soluble tetrazolium salt solution (Cell Counting Kit-8) was purchased from Dojindo for cell viability and proliferation assays. The 1H nuclear magnetic resonance (NMR) spectrum of the synthesized copolymer was obtained using a Mercury NMR spectrometer system (400 MHz) from Varian. The molecular weight of the polymer was analyzed using a YL9100 gel permeation chromatography (GPC) system from YL Instruments equipped with an analytic column with aqueous stationary phases (Ultrahydrogel 1000) from Waters, using poly(ethylene oxide) standards and an aqueous sodium nitrate solution (0.2 M) as the mobile phase. The elemental composition of the polymer surface was assessed using an X-ray photoelectron spectrometer system (K-Alpha+) from Thermo Scientific. A plate photometer (Multiskan FC) from Thermo Scientific was used for colorimetric assays. An inverted microscope (Eclipse Ts2-FL) equipped with a color camera (DS-Fi3) and imaging software (NIS-Elements) from Nikon were utilized to collect and analyze microscopic images and the routine culture of cells. A motorized force tester (MultiTest-dV) from Mecmesin was used to measure compression forces.Preparation of the injectable bulking agents
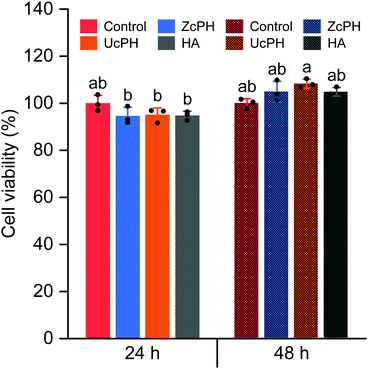
In vitro biocompatibility of the injectable bulking agents
In vivo biocompatibility tests of the injectable bulking agent
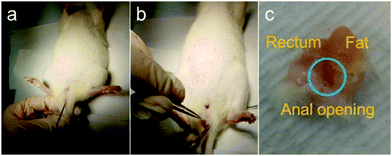
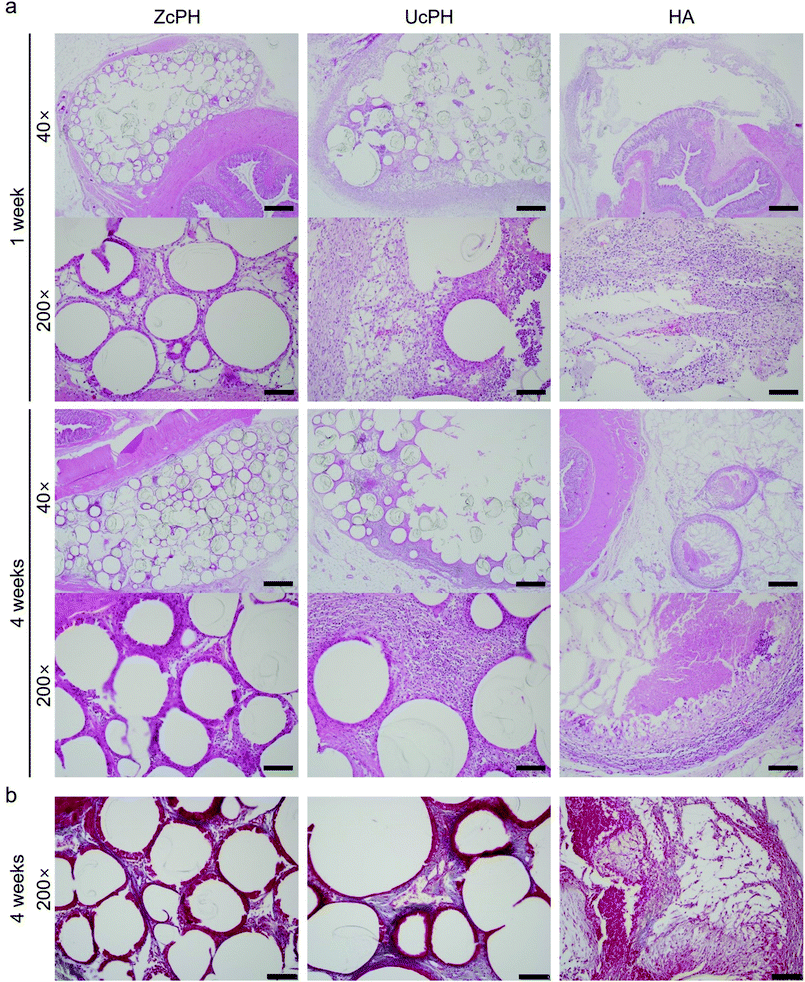
Three animals per group were sacrificed at intervals of 1 week and 4 weeks after the injection treatment. The rats were anesthetized using inhaled isoflurane and killed with concentrated CO2 gas in the designated euthanasia chamber. The anus, perianal region, and rectum were removed together to protect the condition of the injected materials (Fig. 1b and c)
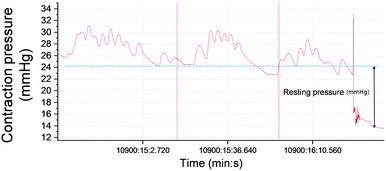
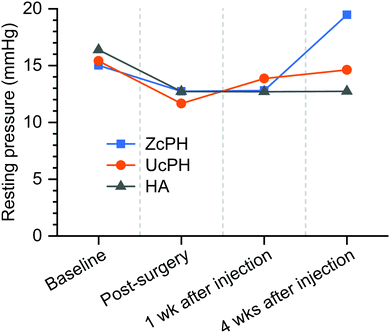
The lungs, liver, kidney, and iliac lymph node were also excised. A manometric evaluation was only performed on the 9 rats that were sacrificed 4 weeks after injection treatment. Manometry was taken at baseline, after the sphincter injury, and 1 week and 4 weeks after injection treatment.
All experimental procedures were approved by the Animal Ethics Committee of Korea University College of Medicine (IRB No. KOREA-2019-0037).
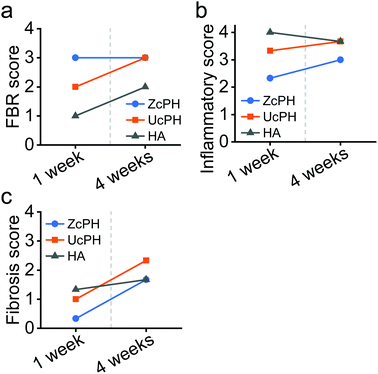
Microscopic evaluation of the local immune reaction at the perianal injection site and the systemic migration of the implant to the distal organs was conducted blind by one pathologist (J.-W. Choi). When evaluating the local immune response, the number of cells per high-power field (×400) around the implant was counted and assigned FBR, inflammatory, and fibrosis scores. Each score was measured as the average of 3 different areas, which was estimated to be the highest score in the low-power field (×100).
The FBR score reflects the immune response sequence based on the severity of the inflammatory reaction and the extent of tissue repair.16 The inflammatory score measures the acute inflammatory response and is divided into 5 stages from 0 to 4 depending on the severity of the inflammation. The inflammatory cells counted are polymorphonuclear cells, lymphocytes, eosinophils, and plasma cells. The fibrosis score indicates the severity of fibrosis in the tissue repair process and is also divided into 5 stages from 0 to 4 depending on the thickness of the fibrotic band. (Table 1). The systemic migration of injected materials was assessed by identifying whether there were any foreign materials or an inflammatory reaction in distant organs.
| Category | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| a Polymorphonuclear cells, lymphocytes, eosinophils, and plasma cells are counted. b The FBR score, established by Duranti et al., represents the immune response sequence based on the severity of the inflammatory reaction and the extent of tissue repair.16HPF, high power field (× 400); FBR, foreign body reaction. | |||||
| Inflammatory scorea | 0 | Rare, 1–10/HPF | 10–30/HPF | Heavy infiltrate | Packed |
| Fibrosis score | 0 | Narrow band | Moderately thick band | Thick band | Extensive band |
| FBR scoreb | 0 | Slight reaction with a few inflammatory cells | Clear inflammatory reaction with 1 or 2 giant cells | Fibrous tissue with inflammatory cells and giant cells | Granuloma with encapsulated implants—clear FBR |
Results
Preparation of the injectable bulking agents
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × 104 and the molecular-weight dispersity (ĐM) was 3.93 (ESI† Fig. S2).
× 104 and the molecular-weight dispersity (ĐM) was 3.93 (ESI† Fig. S2).
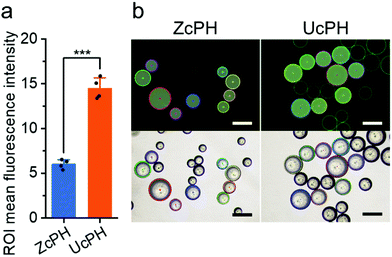
In vitro biocompatibility of the injectable bulking agent
Animal tests
The rats did not exhibit any abnormal behavior or signs of infection during the experimental period. The perianal lesions also appeared generally normal.Discussion
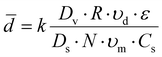 | (1) |
![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) is the average particle diameter, k is the apparatus design factors, Dv is the diameter of the vessel, Ds is the diameter of the stirrer, R is the volume ratio of the dispersed phase to the suspension medium, N is the stirring speed, υd is the viscosity of the dispersed phase, υm is the viscosity of the suspension medium, ε is the interfacial tension between the 2 immiscible phases, and Cs is the stabilizer concentration.
is the average particle diameter, k is the apparatus design factors, Dv is the diameter of the vessel, Ds is the diameter of the stirrer, R is the volume ratio of the dispersed phase to the suspension medium, N is the stirring speed, υd is the viscosity of the dispersed phase, υm is the viscosity of the suspension medium, ε is the interfacial tension between the 2 immiscible phases, and Cs is the stabilizer concentration.
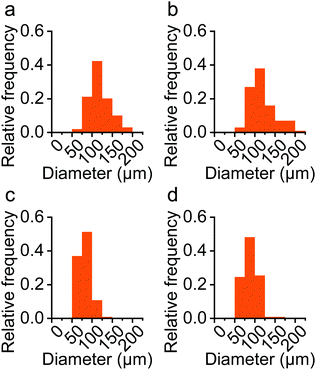
The present study attempted to control the size of the particles by varying the volume ratio of the 2 phases (R) and the stirring speed (N) because these factors are easily controllable.23 The reduction in the particle diameter at a higher stirring speed was reflective of Equation 1. The distribution was also narrower for the smaller particles, which is in line with a previous report.24 However, R did not significantly affect the particle diameter. Because the volume ratio may have a limited effect on the particle sizes in a given system,25 it appears that the volume ratio in this study exceeded the critical ratio for size tunability. The volume fractions of PDMS employed in this study were favorable for high productivity,25 given that bulking agents in which PDMS particles account for 40% of the total weight were required. Overall, the results demonstrated that the particle size was adjustable to some extent by simply altering a single variable. For the in vivo injection purposes, the results confirmed that the mean particle size exceeded 118 μm to avoid phagocytosis and systemic migration. After being mixed with HA gel carriers, the injectability was investigated. The particles were not injectable without HA carriers while suitable injectability of the injection force under 0.9 N through a syringe with an 18G needle was confirmed with HA gel carriers (ESI† Fig. S5). With further optimization employing sophisticated reactor designs, strong quality control, such as high uniformity, could be achieved. Thus, it is expected that the size tunability of this method offers a wider range of potential applications that are in compliance with the requirements of injectable bulking agents.
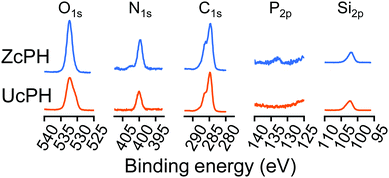
Materials that have been subject to zwitterionic treatment include PET, PC-based urethanes, Ti alloys, PTFE, and PDMS.26–29 Of these materials, PDMS has received significant attention because of its softness as an elastomer, which facilitates mechanical adaptation to the surrounding tissue.30 However, it is well-known that the hydrophobic recovery of the PDMS surface limits long-term functionality.31 This interfacial phenomenon results from polymer chain rearrangements that minimize the difference in surface energy at the contact between the hydrophobic air and transiently functionalized PDMS surface.32 In the present study, the zwitterion-coated PDMS particles were encapsulated in HA carrier gels. In this way, it is believed that the functionalization of the PDMS surface was maintained for a period of time until the protein adsorption tests were conducted. The independent trials in the protein adsorption tests included data collected 1 month after the preparation of the materials. This could also be a proxy for assessing the stability of the modified surfaces and provided a rationale for the following in vivo biocompatibility tests in a rat model.
In accordance with other reports, the FBR in the local tissue in our rat model was mild, with multinucleated giant cells, macrophages, fibroblasts, and collagen deposits.18 This FBR did not cause tissue destruction, thus implants using PDMS and/or HA materials appear to have potential for use in medical applications. Semi-quantitative histological scores were used to compare the local immunological response between the materials. The FBR score for the ZcPH and UcPH groups was similar during the final follow-up but, when the immune response was separated into the early inflammatory reaction and the late fibrotic reaction, the ZcPH group exhibited a weaker reaction at both points of the observed period compared to the UcPH group.
The inflammatory score reflects the early FBR, which usually reaches a peak 1–4 weeks after implantation.6 Our experimental period was thus sufficient to investigate this indicator. During this period, the ZcPH group had a lower inflammatory score than the HA group, although this was not statistically significant. This may suggest that zwitterionic-coated surfaces prevent protein adsorption and consequently reduce the inflammatory reaction.
The fibrosis score reflects the late FBR. Although the exact mechanisms underlying the FBR remain unclear, fibrotic activity can be affected by chronic inflammatory stimuli driven by the macrophages and fibroblasts surrounding the implants. This differs from normal tissue healing and can be as persistent as the tissue repair process.6,37 The formation of a thick fibrotic capsule around implants is considered a critical step as a means to avoid phagocytosis, increase the volume of the implant, and prevent local migration within the tissue plane.12,14 However, Harlim et al. recently classified the FBR of silicone implants into mild or severe forms depending on the degree of fibrosis and suggested that, when an inflammatory response was followed by mild fibrosis, implants would be more preserved and the surrounding tissue would be less damaged.37 We also believe that the excessive formation of fibrotic capsules between implants and tissue can disturb the integration of the implant and reduce its long-term function.
It is possible that the experimental period was too short to fully investigate the fibrotic process associated with the implants. Given that the ZcPH group had a lower fibrosis score than the UcPH group at both follow-up points, continuous experiments could be performed to evaluate the possibility that zwitterionic-coated PDMS can help to attenuate chronic inflammation and reduce fibrotic activity. In particular, further studies investigating changes in the inflammatory mediators and the extracellular matrix around the implants are crucial. No signs of foreign materials or inflammation were observed in the resected distant organs. Most PDMS particles in the filler exceeded 100 μm in diameter, hence systematic migration was expected to be inherently unlikely. However, this also needs to be confirmed via repeated experiments at different injection sites.
Conclusions
In conclusion, a newly proposed tissue bulking agent was tested for the treatment of FI in a preclinical rat model. A zwitterionic polymer was employed for the surface treatment of PDMS, and the fabrication process was controlled to improve the biocompatibility, injectability, and size tunability of the resulting bulking agent. Although statistical differences were not derived from in vivo experiments, the ZcPH showed a tendency to maintain a higher anal resting pressure 4 weeks after treatment and produced better histological scores for the local immune reaction compared to the UcPH bulking agent. In spite of the fact that investigations using larger animal models with longer follow-up periods are required to confirm these findings, the results indicate that the low durability of bulking agent therapy, which has been identified as an inherent drawback of this therapy, can be improved with the suitable selection and tuning of the bulking materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by a grant from Korea University College of Medicine and Korea University Ansan Hospital. This research was also supported by ATC+ Program (20014029) funded by Korea Evaluation Institute of Industrial Technology.References
- A. K. Macmillan, A. E. Merrie, R. J. Marshall and B. R. Parry, Dis. Colon Rectum, 2004, 47, 1341–1349 CrossRef PubMed.
- H. Damon, L. Henry, X. Barth and F. Mion, Dis. Colon Rectum, 2002, 45, 1445–1450 CrossRef PubMed.
- K. D. Hong, J. S. Kim, W. B. Ji and J. W. Um, Tech. Coloproctol., 2017, 21, 203–210 CrossRef CAS PubMed.
- Y. Maeda, S. Laurberg and C. Norton, Cochrane Database Syst. Rev., 2013, CD007959, DOI:10.1002/14651858.CD007959.pub3.
- E. Fournier, C. Passirani, C. N. Montero-Menei and J. P. Benoit, Biomaterials, 2003, 24, 3311–3331 CrossRef CAS PubMed.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- J. B. Schlenoff, Langmuir, 2014, 30, 9625–9636 CrossRef CAS PubMed.
- B. Cao, Q. Tang and G. Cheng, J. Biomater. Sci., Polym. Ed., 2014, 25, 1502–1513 CrossRef CAS PubMed.
- Y. Takatori, T. Moro, M. Kamogawa, H. Oda, S. Morimoto, T. Umeyama, M. Minami, H. Sugimoto, S. Nakamura, T. Karita, J. Kim, Y. Koyama, H. Ito, H. Kawaguchi and K. Nakamura, J. Artif. Organs, 2013, 16, 170–175 CrossRef CAS PubMed.
- Y. Yamada, S. Saito, T. Nishinaka and K. Yamazaki, ASAIO J., 2012, 58, 402–406 CrossRef PubMed.
- Z. Cao, L. Zhang and S. Jiang, Langmuir, 2012, 28, 11625–11632 CrossRef CAS PubMed.
- T. F. Carroll, A. Christie, M. Foreman, G. Khatri and P. E. Zimmern, Int. Braz. J. Urol., 2019, 45, 989–998 CrossRef PubMed.
- C. Sittel, W. F. Thumfart, C. Pototschnig, C. Wittekindt and H. E. Eckel, J. Biomed. Mater. Res., 2000, 53, 646–650 CrossRef CAS PubMed.
- M. M. Soerensen, L. Lundby, S. Buntzen and S. Laurberg, Colorectal Dis., 2009, 11, 73–76 CrossRef CAS PubMed.
- L. Brügger, R. Inglin, D. Candinas, T. Sulser and D. Eberli, Int. J. Colorectal Dis., 2014, 29, 1385–1392 CrossRef PubMed.
- F. Duranti, G. Salti, B. Bovani, M. Calandra and M. L. Rosati, Dermatol. Surg., 1998, 24, 1317–1325 CAS.
- J. A. Champion, A. Walker and S. Mitragotri, Pharm. Res., 2008, 25, 1815–1821 CrossRef CAS PubMed.
- P. H. Nijhuis, T. E. Van Den Bogaard, M. J. Daemen and C. G. Baeten, Dis. Colon Rectum, 1998, 41, 624–629 CrossRef CAS PubMed.
- M. Busso and R. Voigts, Dermatol. Surg., 2008, 34, S16–S24 CAS.
- S. M. Zeitels, P. J. Lombardo, J. L. Chaves, W. C. Faquin, R. E. Hillman, J. T. Heaton and J. B. Kobler, Ann. Otol., Rhinol., Laryngol., 2019, 128, 71S–81S Search PubMed.
- S. S. Johl and R. A. Burgett, Curr. Opin. Ophthalmol., 2006, 17, 471–479 CrossRef PubMed.
- G. Polacco, M. Palla and D. Semino, Polym. Int., 1999, 48, 392–397 CrossRef CAS.
- R. Arshady, Colloid Polym. Sci., 1992, 270, 717–732 CrossRef CAS.
- C. Kotoulas and C. Kiparissides, Chem. Eng. Sci., 2006, 61, 332–346 CrossRef CAS.
- J. Alvarez, J. Alvarez and M. Hernandez, Chem. Eng. Sci., 1994, 49, 99–113 CrossRef CAS.
- X. Jin, J. Yuan and J. Shen, Colloids Surf., B, 2016, 145, 275–284 CrossRef CAS PubMed.
- M. Khan, J. Yang, C. Shi, Y. Feng, W. Zhang, K. Gibney and G. N. Tew, Macromol. Mater. Eng., 2015, 300, 802–809 CrossRef CAS.
- Y.-H. Gu, H.-W. Liu, X.-H. Dong, Z.-Z. Ma, Y.-X. Li, L. Li, D.-L. Gan, P.-S. Liu and J. Shen, Rare Met., 2022, 41, 700–712 CrossRef CAS.
- N. Li, T. Li, X.-Y. Qiao, R. Li, Y. Yao and Y.-K. Gong, ACS Appl. Mater. Interfaces, 2020, 12, 12337–12344 CrossRef CAS PubMed.
- A. Victor, J. Ribeiro and F. S. Araújo, J. Mech. Eng. Biomech., 2019, 4, 1–9 CrossRef.
- H. Zhang and M. Chiao, J. Med. Biol. Eng., 2015, 35, 143–155 CrossRef PubMed.
- A. Gokaltun, M. L. Yarmush, A. Asatekin and O. B. Usta, Technology, 2017, 05, 1–12 CrossRef PubMed.
- V. Cannella, R. Altomare, V. Leonardi, L. Russotto, S. Di Bella, F. Mira and A. Guercio, BioMed Res. Int., 2020, 2020, 8676343 Search PubMed.
- C. H. Jeong, D. H. Kim, J. H. Yune, H. C. Kwon, D.-M. Shin, H. Sohn, K. H. Lee, B. Choi, E. S. Kim, J. H. Kang, E. K. Kim and S. G. Han, Toxicol. In Vitro, 2021, 70, 105034 CrossRef CAS PubMed.
- K. De Boulle, R. Glogau, T. Kono, M. Nathan, A. Tezel, J.-X. Roca-Martinez, S. Paliwal and D. Stroumpoulis, Dermatol. Surg., 2013, 39, 1758–1766 CrossRef CAS PubMed.
- M. Zutshi, L. B. Salcedo, P. J. Zaszczurynski, T. L. Hull, R. S. Butler and M. S. Damaser, Dis. Colon Rectum, 2009, 52, 1321–1329 CrossRef PubMed.
- A. Harlim, M. Kanoko and S. Aisah, Dermatol. Surg., 2018, 44, 1174–1182 CrossRef CAS PubMed.
- K. Ishihara, J. Biomed. Mater. Res., Part A, 2019, 107, 933–943 CrossRef CAS PubMed.
- A. Fallacara, S. Manfredini, E. Durini and S. Vertuani, Facial Plast. Surg., 2017, 33, 87–96 CrossRef CAS PubMed.
- G. Kaya and F. Oytun, Biointerface Res. Appl. Chem., 2021, 11, 8424–8430 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tb02341a |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |