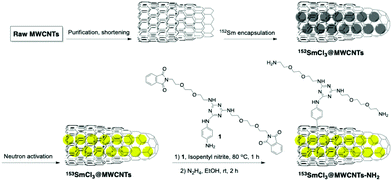
Functionalization of filled radioactive multi-walled carbon nanocapsules by arylation reaction for in vivo delivery of radio-therapy†
Agnieszka
Gajewska‡
a,
Julie T.-W.
Wang‡
b,
Rebecca
Klippstein
b,
Markus
Martincic
c,
Elzbieta
Pach
d,
Robert
Feldman
e,
Jean-Claude
Saccavini
e,
Gerard
Tobias
 c,
Belén
Ballesteros
c,
Belén
Ballesteros
 d,
Khuloud T.
Al-Jamal
d,
Khuloud T.
Al-Jamal
 *b and
Tatiana
Da Ros
*b and
Tatiana
Da Ros
 *a
*a
aINSTM, Trieste Unit & Department of Chemical and Pharmaceutical Sciences, University of Trieste, Via Licio Giorgieri 1, 34127 Trieste, Italy. E-mail: daros@units.it
bSchool of Cancer and Pharmaceutical Sciences, Faculty of Life Sciences & Medicine, King's College London, London SE1 9NH, UK. E-mail: khuloud.al-jamal@kcl.ac.uk
cInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus UAB, 08193 Bellaterra, Barcelona, Spain
dCatalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC and the Barcelona Institute of Science and Technology, Campus UAB, 08193 Bellaterra, Barcelona, Spain
eCis Bio International Ion Beam Applications SA (IBA), 91400 Saclay, France
First published on 19th November 2021
Abstract
Functionalized multi-walled carbon nanotubes (MWCNTs) containing radioactive salts are proposed as a potential system for radioactivity delivery. MWCNTs are loaded with isotopically enriched 152-samarium chloride (152SmCl3), the ends of the MWCNTs are sealed by high temperature treatment, and the encapsulated 152Sm is neutron activated to radioactive 153Sm. The external walls of the radioactive nanocapsules are functionalized through arylation reaction, to introduce hydrophilic chains and increase the water dispersibility of CNTs. The organ biodistribution profiles of the nanocapsules up to 24 h are assessed in naïve mice and different tumor models in vivo. By quantitative γ-counting, 153SmCl3@MWCNTs-NH2 exhibite high accumulation in organs without leakage of the internal radioactive material to the bloodstream. In the treated mice, highest uptake is detected in the lung followed by the liver and spleen. Presence of tumors in brain or lung does not increase percentage accumulation of 153SmCl3@MWCNTs-NH2 in the respective organs, suggesting the absence of the enhanced permeation and retention effect. This study presents a chemical functionalization protocol that is rapid (∼one hour) and can be applied to filled radioactive multi-walled carbon nanocapsules to improve their water dispersibility for systemic administration for their use in targeted radiotherapy.
Introduction
Carbon nanotubes (CNTs) can be categorized as single- and multi-walled carbon nanotubes (SWCNTs and MWCNTs, respectively).1 SWCNTs are characterized as thinner and more reactive on the surface whereas MWCNTs, in contrast to SWCNTs, have remarkably better dispersibility, resistance for processing and higher capacity for internal filling. Generally speaking, both types of CNTs have very promising characteristics, which can be useful for biological applications.2 One very important advantage of CNTs is the ability to cross biological barriers with a small cytotoxic effect.3 This makes them an excellent host material for several therapeutic agents where different parameters can be modified to obtain the best biological effect.4The carbon nanotubes can be subjected to external functionalization by attaching various moieties on the outside of the tubes, but they can also be modified by filling the inner cavity with various compounds, with an endohedral approach. The resulting filled CNTs are described as X@CNTs where X indicates the content of the tube.
In the last decade, rational design and assembly of novel tumor-targeting radiopharmaceuticals has risen under the concept of cancer treatment and diagnosis. In a perfect situation, radiopharmaceuticals should target tumor tissues with small overall loss of radionuclides in the body and supply highest dosage of radiation. With this regard, among other vehicles as calcium carbonate microstructures,5 CNTs have been proposed as model radioactivity carriers to deliver the specific irradiation in cancer-affected tissues and improve the outcome of diagnosis and treatment. A limited number of examples of radioactive CNTs have been reported in the literature so far. For the first time the activity of such system in a biological media was reported on SWCNTs filled with NaI. Hong et al. presented SWCNTs filled with Na125I (Auger and γ-emitter), functionalized and glycosylated for in vitro and in vivo studies.6 The material had a specific tissue accumulation in lungs due the physical properties of the SWCNTs in a physiological context.7 Remarkably, leakage of the radionuclide was not observed in comparison to nanotubes where radioisotopes were externally bounded (86Y, 111In, 14C, 64Cu or 99mTc).8 Pascu et al. have given versatility to the encapsulation approach by sealing 64Cu inside the cavities of SWCNTs.9 The containment of radionuclides was achieved by using fullerenes as corks, and the external walls were non-covalently wrapped with β-D-glucan.
Among several isotopes used in radiotherapy, Spinato et al. tested two radionuclide analogues (SmCl3 and LuCl3) encapsulated in SWCNTs for targeted anticancer therapy. They reported the functionalization of metal halides-filled and sealed nanotubes by nitrene cycloaddition. Conjugation with a monoclonal antibody (Cetuximab) showed improved cancer cell targeting in vitro.10 For both filled SWCNTs, it was observed that the functionalization allowed the active endocytosis and “nanoneedle” mechanism, a passive trans-location into the cytoplasm, previously reported.11 No significant reduction in viability was observed in vitro. This finding is very promising for the application of radioactive carbon nanotubes-based materials because the entrapment of the isotope could prevent its leakage in an uncontrolled manner under complex environment in vivo.
These observations prompted our current work focusing on the application of functionalized MWCNTs as delivery systems for therapeutic and diagnostic radioisotopes. Nanocapsules composed of MWCNTs are likely to offer a higher loading capacity for radioactive isotopes, better resistance for processing and lower uncontrolled release of the radioisotopes in the organism compared to SWCNTs. Functionalization by covalent decoration of their external walls is necessary to provide better dispersibility and biocompatibility and to facilitate subsequent modification with targeting ligands. The choice of the functionalization methodology for such purposes should be carefully selected in such a way that functionalization reaction is highly efficient while keeping its duration minimal, especially important for short-lived radionuclides. The reaction needs to be mild not to damage the walls integrity which may cause premature release of the previously sealed metals. Oxidation-based reaction approaches are naturally excluded. In this study, we propose a modified Tour reaction functionalization approach with triazine units being introduced to the walls of SmCl3 filled-MWCNTs. It was possible to shorten the reaction time down to 1 h, without significantly decreasing the functionalization degree.12 The functionalized MWCNTs were fully characterized prior to studying their bio-interactions with cells and organ biodistribution in different types of tumor-bearing mice. Cytotoxicity of non-functionalized and functionalized nanocapsules at different concentrations was assessed in murine B16F10 melanoma and J774 macrophages. Functionalization was performed on radioactive SmCl3 filled-MWCNTs, irradiated by neutron activation.13 The organ biodistribution profiles were established and tumor uptake were examined up to 24 h in naïve mice and in tumor-bearing mice (GL261 glioma model, B16F10 melanoma implanted subcutaneously and B16F10 melanoma experimental lung metastasis model) by quantitative γ-counting, following intravenous injection.
Results
Filling of MWCNTs with samarium
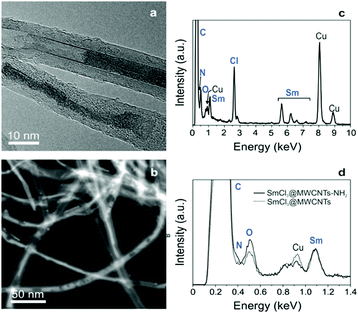
In the first step, MWCNTs were treated by oxidative purification followed by steam treatment to shorten the nanotubes, remove metal and amorphous carbon impurities. TGA of purified MWCNTs were performed under flowing air for quantification of the inorganic impurities in the samples deriving from the synthesis of the nanotubes. The amount of inorganic solid residue after the TGA analysis turned out to be 1.2 wt%. Since iron is used as catalyst for the growth of MWCNTs, the residue after the oxidative analysis will consist of Fe2O3, assuming that no other impurities are present. Therefore, a 1.2 wt% residue corresponds to a 0.8 wt% of iron catalyst in the sample (ESI,† Fig. S1a). MWCNTs underwent endohedral functionalization with samarium chloride (SmCl3) by molten phase high temperature filling. The filled, closed-ended tubes were then purified with acidic water and filtered to remove the non-encapsulated SmCl3. After this step, from TGA results it was possible to calculate the filling yield using a previously reported formula14 and a filling yield of 17 wt% was obtained (Fig. S1b, ESI†). The encapsulation of the metal halides in the internal cavities of MWCNTs was confirmed by electron microscopy imaging. By HAADF STEM imaging, direct visualization of the encapsulated material was achieved and a high degree of filling can be observed (Fig. 1a). The filling appears with a bright intensity whereas the carbon nanotubes are seen in a pale grey contrast.15 The evidence of the confinement of the materials inside MWCNTs was also provided by high resolution TEM imaging (Fig. 1b) where the encapsulated SmCl3 was seen to adopt both nanowire and single-layered nanotube structures, as has been previously reported.16 Further confirmation of the filling was achieved with energy dispersive X-ray spectroscopy (EDX) coupled to the transmission electron microscope. EDX analyses (Fig. 1c) revealed the presence of SmCl3 and the absence of impurities (note that the Cu peak arises from the TEM support grid). | ||
| Fig. 1 (a) HAADF-STEM image of SmCl3@MWCNTs; (b) HRTEM image SmCl3@MWCNTs; (c) SmCl3@MWCNTs EDX spectrum showing the presence of samarium and chlorine atoms. | ||
Functionalization of samarium-filled MWCNTs
The covalent functionalization of filled MWCNTs was carried out via aryl diazonium chemistry.17 The reaction has been reported first by Tour, and later performed by many other authors as a highly reactive pathway for the sidewall decoration of SWCNTs and also MWCNTs12,18 under mild conditions using aniline derivatives. It is postulated that the electron from the CNTs is transferred onto diazonium salt, and then after elimination of N2 a reactive aryl radical is formed.19 Usually, this reaction is performed under heating for long periods but we previously reported an optimized approach with short reaction time (1 h).12 Working with radioactive materials requires to be as fast as possible to minimize the radioactivity decay during the reaction, especially if in presence of short living species. Another crucial aspect of the functionalization to be taken into account is the necessity to minimize the radioactive contaminated waste. The functionalization process was first optimized using cold materials using minimum quantities of solvents, and then applied to the hot 153SmCl3@MWCNTs. The synthesis is described in Scheme 1. In order to enhance the water-dispersibility of filled tubes and to offer functional side chains, an aniline bearing a triazine unit was modified with two aminoethylene glycol chains (Scheme 1). With this approach, it was possible to introduce two solubilizing appendages per aryl unit. The functionalization was initially performed on cold SmCl3@MWCNTs, and later with 152SmCl3@MWCNTs, using a 25-fold mass excess of aniline derivative. The mixture was stirred at 80 °C for 1 h. To functionalize MWCNTs containing γ-emitter 153Sm, a quantity of cold isotopically enriched material was added to the radioactive compound to have a manageable quantity of materials to perform the reaction and the same procedure of functionalization and cleavage was applied on the hot mixture (Scheme 1).Afterwards, the solution was filtered and washed by cycles of ultrasonication and filtration using different solvents to guarantee a clean sample. The phthalimide protecting group was cleaved from SmCl3@MWCNTs-Pht by hydrazine in ethanol.
Characterization of functionalized, filled multi-walled carbon nanotubes
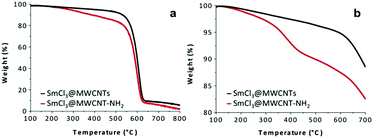 | ||
Fig. 2 TGA profiles of SmCl3@MWCNTs (–) and functionalized SmCl3@MWCNTs-NH2 ( ), (a) in air; (b) in N2 atmosphere. ), (a) in air; (b) in N2 atmosphere. | ||
The degradation process under inert atmosphere was monitored to determine the degree of functionalization obtained using derivative 1 (see Scheme 1), in the temperature ranging between 100 and 700 °C (Fig. 2b). The weight loss observed between 250 and 550 °C was attributed to the degradation of the organic decoration and corresponds to a loading of 90 µmol (triazine derivative) g−1. The free amine content on functionalized SmCl3@MWCNTs-NH2 was determined by colorimetric Kaiser test (60 µmol g−1). In both cases (TGA and Kaiser test), the content of encapsulated salt was taken into account to calculate the functionalization values.
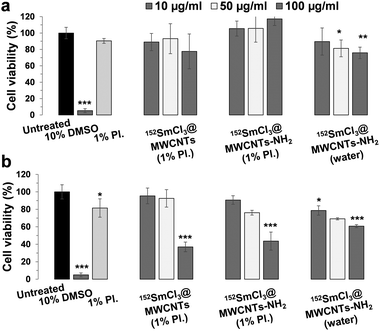
Cytotoxicity of 152SmCl3@MWCNTs in B16F10-Luc and J774 cells
To assess how filled non-radioactive MWCNTs (both non-functionalized and functionalized) can affect cell survival, cytotoxicity tests were performed using melanoma B16F10-Luc and macrophages J774 cells. Both 152SmCl3@MWCNTs and 152SmCl3@MWCNTs-NH2 were dispersed in 1% Pluronic F-127. To better compare the effect of chemical functionalization on cell viability, functionalized 152SmCl3@MWCNTs-NH2 dispersed in water were also prepared for comparison. Cells were incubated with 10, 50 and 100 µg mL−1 of CNTs for 72 h. Cells exposed to 10% DMSO and the surfactant alone were used as controls.The cytotoxicity was determined by the modified LDH assay (Fig. 4 and Fig. S2, ESI†).20 Cells incubated in media containing the same amount of Pluronic F-127 did not show obvious reduction in cell viability (Fig. 4, light grey bars). In the case of B16F10-Luc cells (Fig. 4a), the 72 h-exposure of both CNT types did not cause significant cytotoxicity in most of the tested conditions (>80% cell viability), except treatments of 152SmCl3@MWCNTs-NH2 at higher concentrations (i.e. 50 and 100 µg mL−1). In the case of J774 cells (Fig. 4b), a dose-dependent reduction in cell viability was found with significant toxicity observed at 100 µg mL−1 (p < 0.001). In B16F10-Luc cells, no significant difference was observed between CNTs dispersed in water and in Pluronic F-127. In contrast, as observed in J774 cells, 152SmCl3@MWCNTs-NH2 dispersed in water were less toxic than those dispersed in Pluronic F-127 at 100 µg mL−1 (p < 0.001) (Fig. 4b).
Neutron irradiation
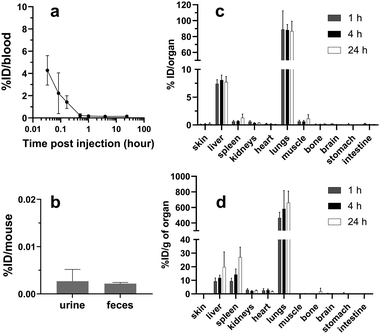
Part of the so-obtained batch of filled 153SmCl3@MWCNT was activated as described above. The specific radioactivity (SRA) values of the sample were measured as 15.97 GBq per mg of 153SmCl3@MWCNTs after neutron irradiation for 78 h.Blood clearance, excretion and organ biodistribution profiles of 153SmCl3@MWCNTs-NH2 in naïve mice
The blood clearance profile of 153SmCl3@MWCNTs after intravenous injection is presented in Fig. 5a. 153SmCl3@MWCNTs displayed fast clearance from the circulation within the first 1 h after injection. To study the excretion profile of the 153SmCl3@MWCNTs-NH2, animals were housed in metabolic cages (one mouse per cage) and the urine and feces were collected at 24 h post-administration followed by γ-counting (Fig. 5b). It was found that negligible amounts (<0.02% of total injected dose) were eliminated into urine and feces.The biodistribution of 153SmCl3@MWCNTs-NH2 in major organs in mice was assessed at 1, 4 and 24 h after intravenous injection. As shown in Fig. 5c, mice treated with 153SmCl3@MWCNTs-NH2 showed the highest uptake in lung (88.8 ± 23.5% ID g−1) followed by liver (7.4 ± 0.8% ID g−1) and spleen (0.9 ± 0.6% ID g−1) at 1 h post-injection. No significant change in the uptake in lung and liver was observed over time.
Organ biodistribution and tumor uptake of 153SmCl3@MWCNTs-NH2 in tumor-bearing mice
The organ biodistribution profiles of 153SmCl3@MWCNTs-NH2 in different tumor models at 1 and 24 h post-injection are shown in Fig. 6 and Fig. S3 (ESI†). No obvious alterations of the biodistribution patterns were observed between naïve and tumor-bearing mice, with highest accumulation found in lung, followed by liver and spleen (Fig. 6a). The comparison of the uptake in cancerous tissues (glioma, s.c. melanoma and experimental lung metastatic melanoma) and the corresponding tissues in naïve mice (i.e. brain and lung) are shown in Fig. 6b. No significant differences were observed in glioma brain (∼0.8% ID g−1 tissue) and lung metastatic melanoma (∼400% ID g−1 tissue) compared to healthy brain and lung, respectively (data from the above biodistribution study in naïve mice). Doses found in s.c. melanoma tumors at 1 h were in the range of ∼0.4% ID g−1 tissue. The uptake in organs decreased from 1 h to 24 h in glioma brains and s.c. tumors but not in lungs. Retention of 153SmCl3@MWCNTs-NH2 was observed in naïve lungs or cancerous lungs (Fig. 6b, bottom graph). It is worth noting that these measurements were done on perfused animals so values in the organs are unlikely to be related to organ contamination with circulating blood.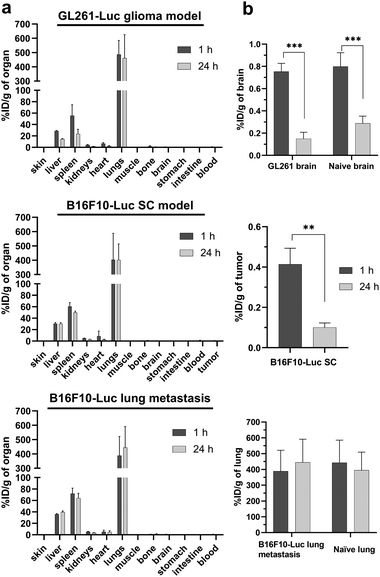 | ||
| Fig. 6 In vivo biodistribution of 153SmCl3@MWCNTs-NH2 in tumour-bearing mice at 1 h and 24 h post-injection. (a) Organ biodistribution profiles in intra-cranial GL261-Luc glioma model (top), subcutaneous B16F10-Luc (B16F10-Luc SC, middle) and B16F10-Luc lung metastasis model (bottom). (b) Uptake in tumours and tumour-containing tissues. Naïve C57BL/6 mice were injected with 200 µg of 153SmCl3@MWCNTs-NH2 containing ∼1.5 MBq. The radioactivity of blood and major organs sampled at 1 h and 24 h post-injection was measured by γ-counting. The results expressed as % ID g−1 of organ and presented as mean ± S.D. (n = 3). The data of tumour accumulation in (b) were extracted from (a) and the data of naïve tissues for comparison were extracted from Fig. 5. Statistical analysis was performed using one way ANOVA with respect to untreated group. **p < 0.01, ***p < 0.001. | ||
Discussion
In the first report on carbon nanocapsules (closed-ended filled carbon nanotubes) for in vivo radioemitter, SWCNTs were filled with Na125I, then covalently functionalized by 1,3-dipolar cycloaddition and glycosylated.6 The study highlighted the specific organ (lung) accumulation and absence of isotope leakage from the GlcNAcD-Na125I@SWCNTs which allowed for non-invasive imaging as well as precise localization of a highly concentrated “radiodose”. More recently, the in vivo fate of Na125I@SWCNTs externally functionalized with other glycans has also been explored.7 However, the functionalization strategy employed in these previous studies required a 96 h reflux of the reaction mixture. This was possible because the employed radionuclide (125I) had a half-life of 59.5 days. However, the majority of clinically employed radionuclides have much shorter half-lives and therefore the chemical functionalization method and sub-sequent purification steps are considered to be lengthy for this application, where shortening step time is very important to minimize chance of isotope decay. Towards this end, non-covalent functionalization with β-D-glucan has been employed for 64Cu@SWCNTs.9 Herein we have developed a fast, scalable and efficient covalent strategy for the functionalization of ‘hot’ nanocapsules in which the functionalization requires 1 h followed by easy and fast work up. The deprotection of Fmoc groups takes place in 2 h, with a total of maximum 4 h for the complete functionalization and collection of the desired products.When it comes to filling strategies, in early studies the filling method employed relied on the use of ‘hot’ (radioactive) material. Taking into account the time constraints imposed by the use of short-lived radionuclides, we have recently developed a modified method by initially filling the inner cavity of the tubes with cold material, 152Sm enriched SmCl3, that can then be neutron irradiated to yield 153SmCl3.13 Additionally, to improve the filling yield further, MWCNTs were employed for radioemitter delivery. MWCNTs have a larger inner diameter thus a higher capacity for internal loading, and a better chance for delivery of higher dose of isotope compared to SWCNTs.3,21 Furthermore, MWCNTs present a higher resistance to structural damage by neutron irradiation than their single-walled counter parts.13 The specific activity of the resulting 153SmCl3@MWCNTs is much higher than the ones previously reported for 125I@SWCNTs and 64Cu@SWCNTs allowing the injection of a smaller dose of tubes with higher radioactivity.
Combined with the previous efforts to improve the specific radioactivity of the filled nanocapsules, this work offers the added advantage of developing a chemical functionalization method that is sufficiently quick to allow functionalization of nanocapsules with short half-lived emitters so the radionuclides retain most of their radioactivity. In fact, starting from the well-known Tour procedure, it was possible to optimize the reaction shortening the time down to 1 h, without significantly decreasing the functionalization degree. The first attempt in this direction, to enhance the performance, was recently reported by us and used as guideline for this work,12 while the usually reported procedures for Tour reaction are overnight17a,c and other successful methodologies can take overnight as well,22 or even days.23 The latter are obviously not applicable in the present case, taking into account the necessity to exploit as much as possible the 153Sm radioactivity, the half-life of which is 46.3 h, as already mentioned. Using the optimized Tour reaction, it was possible to chemically functionalize CNT walls with a linker that decreases aggregation thanks to the free amine functional groups, which are also available for further binding with targeting molecules.
The cytotoxicity experiments were performed using J744 cells, a phagocytic cell line and B16F10-Luc cells, one of the cell lines used as tumor models in vivo. Cells were treated with non-functionalized and functionalized cold filled nanocapsules, dispersed in 1% Pluronic solution or water to evaluate the effect of CNT functionalization and presence of surfactant on cell viability.
To properly suspend the non-functionalized nanocapsules and to use them in the cytotoxicity test and in the in vivo analyses, the presence of Pluronic was necessary. Taking this into account, the SmCl3@MWCNTs-NH2 suspensions were prepared both without and with the surfactant, to be able to properly correlate the results to the carbon nanomaterials avoiding any external interference, and to be able at the same time to correlate the in vivo behavior of the non-functionalized and the functionalized nanocapsules in the same conditions.
No obvious cytotoxicity was induced when cells were treated with all preparations at the lower concentration (10 µg mL−1) up to 72 h. At higher concentrations, B16F10-Luc cells were less affected than macrophages by these treatments. This could be due to the phagocytic nature of J774 cells that more materials were taken up by cells than B16F10-Luc cells.24
In our study, we investigated the in vivo biodistribution profiles of 153SmCl3@MWCNTs-NH2 (Fig. 5 and 6, and Fig. S3, ESI†), functionalized with alternative aryl diazonium chemistry, to provide new platform for further targeting purposes. By quantitative gamma counting, the results indicated high affinity to lung for up to 24 h in naïve mice and diseased tumor models. Moreover, small amount of 153SmCl3@MWCNTs was detected in blood after injection (e.g. ∼4% ID measured at 2 min post-injection) with a complete clearance from blood after 1 h. The absence of isotope in blood, urine and feces after 24 h suggests no-leakage from the tubes and high affinity to the organs.
It is also noted that 153SmCl3@MWCNTs-NH2 seemed to be able to reach brain, at early time post-injection (i.e. ∼0.75% ID g−1 of brain detected at 1 h) (Fig. 6a). The brain uptake property of functionalized MWCNTs undergone different chemical approaches compared to the current study has been reported previously.25,26 The feature of initial brain uptake of the studied 153SmCl3@MWCNTs-NH2 after injection provides another possible future application of delivering radioactivity to treat brain tumors.
It has been reported that SmCl3-filled SWCNTs, conjugated with a targeting ligand, were able to improve the internalization into cancer cells in vitro.10 Future work will focus on developing targeted 153SmCl3@MWCNTs-NH2 to achieve active targeting toward cancer.
Experimental
Materials
Solvents used for synthesis were analytical grade and were purchased from Aldrich, Acros, and Alfa Aesar. When anhydrous conditions were required, high quality commercial solvents were used (THF, DCM, toluene, DMF). Deuterated solvents were from Aldrich and Cambridge Isotope Laboratories. Water was purified using a Millipore filter system MilliQ®. Elicarb® MWCNTs were provided by Thomas Swan & Co. Ltd. MWCNTs were produced by the catalytic chemical vapor deposition process and supplied as a dry powder. Sm2O3 (with natural isotopic composition) was purchased from Sigma-Aldrich and enriched 152Sm2O3 from Eurisotop. Nanotubes filtrations were performed using Millipore JHWP filters, pore size 0.45 µm. Kaiser test kit was purchased from Sigma Aldrich. RPMI-1640 media, penicillin/streptomycin, Trypsin/EDTA, and phosphate buffered saline (PBS) were obtained from Gibco, Thermo Fisher Scientific (UK). D-Luciferin potassium salt was from PerkinElmer (UK). Murine melanoma cells B16F10-Luc and murine glioma cells GL261-Luc were purchased from PerkinElmer (UK). Mouse macrophage cells J774 were purchased from ATCC (ATCC® TIB-67™). Fetal bovine serum (FBS) was obtained from First Link UK Ltd. CytoTox 96® Non-Radioactive Cytotoxicity Assay kit was purchased from Promega UK.Preparation of filled MWCNTs (SmCl3@MWCNTs and 152SmCl3@MWCNTs)
As-received MWCNTs were treated with a mixture of nitric/sulfuric acids followed by 1 h steam treatment following a previously described protocol.27 The resulting short open-ended and purified MWCNTs were filled with samarium(III) chloride (SmCl3) by molten phase capillary filling. When using high temperature filling, the ends of the CNTs spontaneously close on cooling thus leading to close-ended CNTs, that we refer to as carbon nanocapsules.13,16 Anhydrous SmCl3 was synthesized starting from Sm2O3.28 MWCNTs were then mixed with the freshly prepared SmCl3 (w/w 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and grinded in an agate mortar and pestle in an argon-filled glovebox. The mixture was sealed under vacuum inside a silica tube. The sample was then annealed in a horizontal furnace at 1200 °C for 12 h, well above the melting point of SmCl3, to allow the formation of carbon nanocapsules. The resulting sample contained filled closed-ended nanotubes and external crystals of SmCl3. The non-encapsulated material was removed by washing the sample in hot acidic water (ca. 200 mL of water containing 3–5 mL of concentrated HCl). MWCNTs were first soaked in the acidic water and the sample was collected after filtration over a polycarbonate membrane (0.2 µm pore size). This was followed by washing the sample in fresh acidic water with constant stirring, at 80 °C for 24 h, repeated three times, with the filtration step in between washings and change of the acidic water. After completing the washing with acidic water, a final step was added, using the same conditions (80 °C, 24 h of stirring), with 200 mL of water without acid. The same protocol was employed for the encapsulation of 152-samarium(III) chloride (152SmCl3), using isotopically enriched 152Sm2O3 as starting material. The non-irradiated filled MWCNTs are referred to as 152SmCl3@MWCNTs.
10) and grinded in an agate mortar and pestle in an argon-filled glovebox. The mixture was sealed under vacuum inside a silica tube. The sample was then annealed in a horizontal furnace at 1200 °C for 12 h, well above the melting point of SmCl3, to allow the formation of carbon nanocapsules. The resulting sample contained filled closed-ended nanotubes and external crystals of SmCl3. The non-encapsulated material was removed by washing the sample in hot acidic water (ca. 200 mL of water containing 3–5 mL of concentrated HCl). MWCNTs were first soaked in the acidic water and the sample was collected after filtration over a polycarbonate membrane (0.2 µm pore size). This was followed by washing the sample in fresh acidic water with constant stirring, at 80 °C for 24 h, repeated three times, with the filtration step in between washings and change of the acidic water. After completing the washing with acidic water, a final step was added, using the same conditions (80 °C, 24 h of stirring), with 200 mL of water without acid. The same protocol was employed for the encapsulation of 152-samarium(III) chloride (152SmCl3), using isotopically enriched 152Sm2O3 as starting material. The non-irradiated filled MWCNTs are referred to as 152SmCl3@MWCNTs.
Neutron activation of 152SmCl3@ MWCNTs
To maximize the activation of the materials, the MWCNTs were filled with 152Sm enriched samarium chloride and then activated in a neutron flux. The neutron activation was performed at the atomic reactor in Saclay (France). In brief, a silica ampoule containing 15 mg of 152SmCl3@MWCNTs was irradiated at a neutron flux of 1 × 104 n cm−2 s−1 for 78 h. The ampoule was allowed to cool down and the radioactivity was measured using a dose calibrator (VDC 404, Veenstra Instruments, the Netherlands). The ampoule was then shattered, and the now radioactive filled MWCNT (153SmCl3@MWCNTs) powders were suspended in DMF. The so-obtained 153Sm decays by emission of weak γ-rays and β-particles, with a half-life of 46.3 h.Functionalization of SmCl3@MWCNTs and 152SmCl3@ MWCNTs
Due to the high cost of the isotopically enriched material, the functionalization of filled MWCNTs was initially optimized using SmCl3 with natural isotopic composition. SmCl3@MWCNTs (30 mg) were dispersed in 15 mL of DMF by sonication for 5 min. Then compound 1 (see Scheme 1 and Scheme S1, ESI,† 750 mg, 0.88 mmol) was added in 15 mL of DMF, dispersed for another 5 min and cooled to 0 °C (Scheme 1). Isopentyl nitrite (705 µL, 5.1 mmol) was added and reaction mixture was heated up to 80 °C and stirred for 1 h. Then, the cooled mixture was recovered by filtration, washed with DMF until the eluted solvent was colorless, re-dispersed in DMF (×2), filtered, washed with water, MeOH, EtOAc, Et2O and dried under vacuum.For the cleavage of amine protecting groups, 30 mg of the CNTs were dispersed in EtOH (27 mL) by sonication for 10 min, and afterwards treated with hydrazine hydrate (3 mL). The dispersion was stirred at r.t. for 2 h, and then diluted with EtOH (15 mL) and filtered. After filtration, MWCNTs were re-precipitated in EtOH, filtered, washed with 0.1 M HCl solution, water, MeOH, diethyl ether finally dried under vacuum to afford functionalized SmCl3@MWCNTs bearing free amino groups (SmC3@MWCNTs-NH2). The same protocol was performed starting with 152SmCl3@MWCNTs, and the resulting functionalized sample is referred to as 152SmCl3@MWCNTs-NH2.
Functionalization of 153SmCl3@ MWCNTs
The functionalization of the neutron activated 153SmCl3@MWCNTs was performed in a radiolab at King's College London. The specific activity obtained prior to chemical functionalization was 2.11 GBq mg−1 of CNTs. In this experiment, 6 mg of cold 152SmCl3@MWCNTs (1 mg mL−1) were mixed with 45 µg of radioactive 153SmCl3@MWCNTs with the total mixture containing roughly 100 MBq. The sample was dispersed by sonication for 5 min and the procedure of functionalization was followed as described above for the cold materials. After deprotection, the obtained material was re-precipitated in EtOH, filtered, washed with 0.1 M HCl solution and water. Radioactivity of aliquots sampled before and after the functionalization was measured using γ-counting (LKB Wallac 1282 Compugamma, PerkinElmer). The % recovery was measured as 49.2%, indicating half of the radioactivity was lost during the different processing and centrifugation steps. The presence of the free 153Sm in the samples was examined by thin layer chromatography (TLC) followed by γ-counting. No free 153Sm was present in the solvent front, indicating all radioactive 153Sm remained sealed in the cavity of the functionalized MWCNTs. The functionalized 153SmCl3@MWCNTs, referred to as 153SmCl3@MWCNTs-NH2, were directly used for biodistribution studies.Characterization of functionalized SmCl3@MWCNTs-NH2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). Reported values are an average of two separate measurements. UV-vis spectra were recorded on a Cary 5000 spectrophotometer (Varian) using 1 cm path quartz cuvettes main paragraph text follows directly on here.
000 M−1 cm−1). Reported values are an average of two separate measurements. UV-vis spectra were recorded on a Cary 5000 spectrophotometer (Varian) using 1 cm path quartz cuvettes main paragraph text follows directly on here.
Cell toxicity assays
B16F10-Luc melanoma cells and J774 macrophage cells were cultured in Advanced RPMI-1640 media supplemented with 10% FBS, 100 IU mL−1, penicillin, and 100 µg mL−1 streptomycin at 37 °C in 5% CO2 atmosphere.Cells were seeded at a density of 8000 cells per well in flat-bottomed 96-well plates and left to adhere overnight. The 152SmCl3@MWCNTs and 152SmCl3@MWCNTs-NH2 were dispersed in 1% Pluronic F-127 in saline (0.9% NaCl) at 1 mg mL−1. The functionalized material was also dispersed in water without the presence of the surfactant. Cells were then treated with MWCNTs dispersions diluted in complete media at final concentrations of 10, 50, 100 µg mL−1 for 72 h. Cells incubated with complete media containing 10% DMSO were used as a positive control. To avoid risk of interference from the intrinsic absorbance of CNTs at 490 nm, the modified version of the LDH protocol was used to assess the cytotoxicity.20Eqn (1) was used to calculate the cells survival with modified LDH assay:
 | (1) |
Biodistribution study of 153SmCl3@MWCNTs-NH2 in naïve mice by γ-counting
All in vivo experiments were conducted under the authority of project and personal licenses granted by the UK Home Office and the UKCCCR Guidelines (1998). Female C57BL/6 mice aged 6–8 weeks were purchased from Envigo (UK) and used for all in vivo studies.Tissue biodistribution studies of 153SmCl3@MWCNTs-NH2 including assessments of blood circulation and excretion profiles in mice were performed. The radioactive 153SmCl3@MWCNTs-NH2 were re-suspended in 1% Pluronic F-127 saline solution for injection to mice. The final concentration was approx. 1 mg mL−1, containing ∼1.5 MBq per 200 µL. Mice were given 200 µg of 153SmCl3@MWCNTs-NH2 intravenously, via a tail vein. To obtain the excretion profile, mice were housed singly in metabolic cages in which animals had free access to water but not food. After 24 h, urine and feces were collected from individual cages and measured by γ-counting. For blood profiles, blood samples were collected in heparinized capillaries from 4 min up to 24 h after injection and measured by γ-counting. At 1, 4, and 24 h after collecting the blood, the animals (n = 3) were perfused with 25 mL of heparinized saline (50 IU mL−1) via the left ventricle of the heart to clear 153SmCl3@MWCNTs-NH2 remaining in blood. Major organs including skin, liver, spleen, heart, lung, muscle, bone, brain, stomach and intestine were excised and weighed, and the radioactivity was measured by γ-counting. The percentages of injected doses per organ (% ID per organ) and per gram of tissue (% ID g−1 of organ) were calculated for each organ.
Biodistribution study of 153SmCl3@MWCNTs-NH2 in tumor-bearing mice by γ-counting
Organ biodistribution profiles of 153SmCl3@MWCNTs-NH2 were determined in three types of in vivo tumors, all implanted in C57BL/6 mice. To establish subcutaneous (s.c.) B16F10-tumor model, mice were inoculated with B16F10-Luc cells (106 cells in 0.1 mL of PBS) at both flanks. Experimental lung metastatic model was established by intravenous injection of B16F10-Luc cells (5 × 105 cells in 0.2 mL of PBS). Intracranial GL261 glioma model was established by stereotactically-guided injection of 1.25 × 105 GL261-Luc glioma cells (3 µL) into the right hemisphere using a Hamilton syringe (Harvard Apparatus, UK) with a 26-gauge needle at 0.2 µL min−1.25 Tumor growth monitoring was carried out by caliber measurement (for s.c. tumors) or bioluminescence imaging (for lung metastases and glioma tumors) using an IVIS Lumina III system (PerkinElmer, UK).When tumors reached ∼8 mm in diameter (for s.c. tumors) or at 1–2 weeks post inoculation (for lung metastases and glioma tumors), mice were intravenously injected with 153SmCl3@MWCNTs-NH2 at a dose of 200 µg CNTs in 200 µL 1% Pluronic F-127 saline containing (∼1.5 MBq). Organ (including tumors) biodistribution and blood circulation profiles were examined at 1 and 24 h post as described for naïve mice.
Conclusions
This work reports a rapid method for exohedral functionalization of MWCNTs designed for radioactivity delivery. The optimized aryl diazonium chemistry can be carried out in one hour, offering an adequate level of functionalization sufficient to improve the water-dispersibility of MWCNTs and a platform to introduce subsequent biomolecules. Covalent attachment of triazine derivative though sp2 carbon network modification resulted in no leakage of the encapsulated material, indicating that the inner part of tubes remained intact. In mice, organ biodistribution of the aryl functionalized radioactive MWCNTs showed high accumulation in lung followed by liver and spleen, suggesting the suitability to passively target these tumor types in future efficacy studies in animals.Author contributions
AG: investigation, visualization, methodology, validation, writing – original draft. JT-WW: investigation, formal analysis, visualization, validation, writing – original draft. RK: investigation, formal analysis, visualization. MM: investigation, visualization. EP: investigation, visualization. RF: investigation. J-CS: resources, supervision. GT: resources, supervision, funding acquisition, writing – review & editing. BB: resources, supervision, funding acquisition, writing – review & editing. KTA-J: conceptualization, resources, supervision, validation, visualization, funding acquisition, writing – review & editing. TDR: conceptualization, resources, supervision, validation, visualization, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Thomas Swan & Co. Ltd for supplying the Eli-carb® MWCNTs. This work was supported by European Union's Seventh Framework Programme FP7, Project “RADDEL” [grant number 290023], Worldwide Cancer Research [grant number 12-1054], Biotechnology and Biological Sciences Research Council [grant number BB/J008656/1], European Union HORIZON 2020 MSCA RISE 2016, Project Carbo-Immap [grant number 734381], “Severo Ochoa” Programme for Centres of Excellence in R&D [grant numbers SEV-2015-0496, SEV-2017-0706], and Generalitat de Catalunya 2017 [grant number SGR 327].References
- (a) S. Iijima, Nature, 1991, 354, 56 CrossRef CAS; (b) L. Ortolani, F. Houdellier, M. Monthioux and V. Morandi, Carbon, 2010, 48, 3050 CrossRef CAS.
- (a) C. Ménard-Moyon, E. Venturelli, C. Fabbro, C. Samorì, T. Da Ros, K. Kostarelos, M. Prato and A. Bianco, Expert Opin. Drug Discovery, 2010, 5, 691 CrossRef; (b) C. Fabbro, A. Ali-Boucetta, T. Da Ros, K. Kostarelos, A. Bianco and M. Prato, Chem. Commun., 2012, 48, 3911 RSC; (c) A. Battigelli, C. Ménard-Moyon, T. Da Ros, M. Prato and A. Bianco, Adv. Drug Delivery Rev., 2013, 65, 1899 CrossRef CAS PubMed.
- R. Klingeler and R. B. Sim, Carbon nanotubes for biomedical applications, Springer, Berlin, Heidelberg, 2011 Search PubMed.
- (a) A. Vyalikh, A. U. B. Wolter, S. Hampel, D. Haase, M. Ritschel, A. Leonhardt, H.-J. Grafe, A. Taylor, K. Krämer, B. Büchner and R. Klingeler, Nanomedicine, 2008, 3, 321 CrossRef PubMed; (b) M. del Carmen Giménez-López, F. Moro, A. La Torre, C. J. Gómez-García, P. D. Brown, J. van Slageren and A. N. Khlobystov, Nat. Commun., 2011, 2, 407 CrossRef PubMed; (c) M. Martincic and G. Tobias, Expert Opin. Drug Delivery, 2015, 12, 563 CrossRef CAS.
- (a) S. Westrøm, M. Malenge, I. S. Jorstad, E. Napoli, Ø. S. Bruland, T. B. Bønsdorff and R. H. Larsen, J. Labelled Compd. Radiopharm., 2018, 61, 472 CrossRef; (b) M. V. Zyuzin, D. Antuganov, Y. V. Tarakanchikova, T. E. Karpov, T. V. Mashel, E. N. Gerasimova, O. O. Peltek, N. Alexandre, S. Bruyere, Y. A. Kondratenko, A. R. Muslimov and A. S. Timin, ACS Appl. Mater. Interfaces, 2020, 12, 31137 CrossRef CAS.
- S. Y. Hong, G. Tobias, K. T. Al-Jamal, B. Ballesteros, H. Ali-Boucetta, S. Lozano-Perez, P. D. Nellist, R. B. Sim, C. Finucane, S. J. Mather, M. L. H. Green, K. Kostarelos and B. G. Davis, Nat. Mater., 2010, 9, 485 CrossRef CAS.
- S. De Munari, S. Sandoval, E. Pach, B. Ballesteros, G. Tobias, D. C. Anthony and B. G. Davis, Inorg. Chim. Acta, 2019, 495, 118933 CrossRef CAS.
- (a) R. Singh, D. Pantarotto, L. Lacerda, G. Pastorin, C. Klumpp, M. Prato, A. Bianco and K. Kostarelos, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 3357 CrossRef CAS PubMed; (b) M. R. McDevitt, D. Chattopadhyay, J. S. Jaggi, R. D. Finn, P. B. Zanzonico, C. Villa, D. Rey, J. Mendenhall, C. A. Batt, J. T. Njardarson and D. A. Scheinberg, PLoS One, 2007, 2, e907 CrossRef PubMed; (c) X. Deng, S. Yang, H. Nie, H. Wang and Y. Liu, Nanotechnology, 2008, 19, 075101 CrossRef; (d) Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47 CrossRef CAS; (e) A. Ruggiero, C. H. Villa, J. P. Holland, S. R. Sprinkle, C. May, J. S. Lewis, D. A. Scheinberg and M. R. McDevitt, Int. J. Nanomed., 2010, 5, 783 CrossRef CAS PubMed; (f) M. Das, S. R. Datir, R. P. Singh and S. Jain, Mol. Pharmaceutics, 2013, 10, 2543 CrossRef CAS PubMed; (g) J. T.-W. Wang, L. Cabana, M. Bourgognon, H. Kafa, A. Protti, K. Venner, A. M. Shah, J. K. Sosabowski, S. J. Mather, A. Roig, X. Ke, G. Van Tendeloo, R. T. M. de Rosales, G. Tobias and K. T. Al-Jamal, Adv. Funct. Mater., 2014, 24, 1880 CrossRef CAS PubMed; (h) J. T.-W. Wang, C. Fabbro, E. Venturelli, C. Ménard-Moyon, O. Chaloin, T. Da Ros, L. Methven, A. Nunes, J. K. Sosabowski, S. J. Mather, M. K. Robinson, J. Amadou, M. Prato, A. Bianco, K. Kostarelos and K. T. Al-Jamal, Biomaterials, 2014, 35, 9517 CrossRef CAS PubMed; (i) T. Peci, T. J. S. Dennis and M. Baxendale, Carbon, 2015, 87, 226 CrossRef CAS.
- H. Ge, P. J. Riss, V. Mirabello, D. G. Calatayud, S. E. Flower, R. L. Arrowsmith, T. D. Fryer, Y. Hong, S. Sawiak, R. M. J. Jacobs, S. W. Botchway, R. M. Tyrrell, T. D. James, J. S. Fossey, J. R. Dilworth, F. I. Aigbirhio and S. I. Pascu, Chem, 2017, 3, 437 CAS.
- C. Spinato, A. P. Ruiz de Garibay, M. Kierkowicz, E. Pach, M. Martincic, R. Klippstein, M. Bourgognon, J. T.-W. Wang, C. Menard-Moyon, K. T. Al-Jamal, B. Ballesteros, G. Tobias and A. Bianco, Nanoscale, 2016, 8, 12626 RSC.
- (a) D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J.-P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242 CrossRef CAS; (b) Z. Markovic, B. Todorovic-Markovica, D. Kleut, N. Nikolic, S. Vranjes-Djuric, M. Misirkic, L. Vucicevic, K. Janjetovic, L. Harhaji, B. Babic-Stojic, M. Dramicanin and V. Trajkovic, Biomaterials, 2007, 28, 5437 CrossRef CAS PubMed.
- J. M. González-Domínguez, A. Santidrián, A. Criado, C. Hadad, M. Kalbáč and T. Da Ros, Chem. – Eur. J., 2015, 21, 18631 CrossRef PubMed.
- J. T.-W. Wang, R. Klippstein, M. Martincic, E. Pach, R. Feldmann, M. Sefl, Y. Michel, D. Asker, J. Sosabowski, M. Kalbáč, T. Da Ros, C. Menard-Moyon, A. Bianco, I. Kyriakou, D. Emfietzoglou, J.-C. Saccavini, B. Ballesteros, K. T. Al-Jamal and G. Tobias, ACS Nano, 2020, 14, 129 CrossRef CAS.
- B. Ballesteros, G. Tobias, M. A. H. Ward and M. L. H. Green, J. Phys. Chem. C, 2009, 113, 2653 CrossRef CAS.
- M. Martincic, E. Pach, B. Ballesteros and G. Tobias, Phys. Chem. Chem. Phys., 2015, 17, 31662 RSC.
- M. Martincic, S. Vranic, E. Pach, S. Sandoval, B. Ballesteros, K. Kostarelos and G. Tobias, Carbon, 2019, 141, 782 CrossRef CAS.
- (a) J. L. Bahr and J. M. Tour, Chem. Mater., 2001, 13, 3823 CrossRef CAS; (b) J. L. Bahr, J. Yang, D. V. Kosynkin, M. J. Bronikowski, R. E. Smalley and J. M. Tour, J. Am. Chem. Soc., 2001, 123, 6536 CrossRef CAS PubMed; (c) B. K. Price and J. M. Tour, J. Am. Chem. Soc., 2006, 128, 12899 CrossRef CAS PubMed.
- (a) J. Mateos-Gil, L. Rodríguez-Pérez, M. Moreno Oliva, G. Katsukis, C. Romero-Nieto, M. Á. Herranz, D. M. Guldi and N. Martín, Nanoscale, 2015, 7, 1193 RSC; (b) C. Ménard-Moyon, C. Fabbro, M. Prato and A. Bianco, Chem. – Eur. J., 2011, 17, 3222 CrossRef PubMed.
- G. Schmidt, S. Gallon, S. Esnouf, J.-P. Bourgoin and P. Chenevier, Chem. – Eur. J., 2009, 15, 2101 CrossRef CAS.
- H. Ali-Boucetta, K. T. Al-Jamal, K. H. Müller, S. Li, A. E. Porter, A. Eddaoudi, M. Prato, A. Bianco and K. Kostarelos, Small, 2011, 7, 3230 CrossRef CAS.
- R. D. Gately and M. Panhuis, Beilstein J. Nanotechnol., 2015, 6, 508 CrossRef CAS.
- J. T.-W. Wang, C. Spinato, R. Klippstein, P. M. Costa, M. Martincic, E. Pach, A. P. Ruiz De Garibay, C. Menard-Moyon, R. Feldman, Y. Michel, M. Åefl, I. Kyriakou, D. Emfietzoglou, J.-C. Saccavini, B. Ballesteros, G. Tobias, A. Bianco and K. T. Al-Jamal, Carbon, 2020, 162, 410 CrossRef CAS.
- V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger and A. Hirsch, J. Am. Chem. Soc., 2002, 124, 760 CrossRef CAS PubMed.
- P. M. Costa, M. Bourgognon, J. T.-W. Wang and K. T. Al-Jamal, J. Controlled Release, 2016, 241, 200 CrossRef CAS.
- H. Kafa, J. T.-W. Wang, N. Rubio, R. Klippstein, P. M. Costa, H. A. Hassan, J. K. Sosabowski, S. S. Bansal, J. E. Preston, N. J. Abbott and K. T. Al-Jamal, J. Controlled Release, 2016, 225, 217 CrossRef CAS PubMed.
- (a) H. Kafa, J. T.-W. Wang, N. Rubio, K. Venner, G. Anderson, E. Pach, B. Ballesteros, J. E. Preston, N. J. Abbott and K. T. Al-Jamal, Biomaterials, 2015, 53, 437 CrossRef CAS PubMed; (b) J. T.-W. Wang, N. Rubio, H. Kafa, E. Venturelli, C. Fabbro, C. Ménard-Moyon, T. Da Ros, J. K. Sosabowski, A. D. Lawson, M. K. Robinson, M. Prato, A. Bianco, F. Festy, J. E. Preston, K. Kostarelos and K. T. Al-Jamal, J. Controlled Release, 2016, 224, 22 CrossRef CAS PubMed.
- M. Kierkowicz, E. Pach, A. Santidrián, S. Sandoval, G. Gonçalves, E. Tobías-Rossell, M. Kalbáč, B. Ballesteros and G. Tobias, Carbon, 2018, 139, 922 CrossRef CAS.
- M. Martincic, C. Frontera, E. Pach, B. Ballesteros and G. Tobias, Polyhedron, 2016, 116 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures and synthesis details as described in the text. See DOI: 10.1039/d1tb02195h |
| ‡ These authors equally contributed. |
| This journal is © The Royal Society of Chemistry 2022 |