Co, Fe and N co-doped 1D assembly of hollow carbon nanoboxes for high-performance supercapacitors†
Abstract
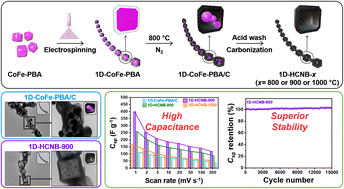
In this study, we successfully demonstrate the synthesis of a novel necklace-like Co, Fe, and N co-doped one-dimensional (1D)-assembly of hollow carbon nanoboxes (1D-HCNB-x) and its potential for a supercapacitor application. The unique hybrid nanoarchitecture of 1D-HCNB-x consisting of hollow zero-dimensional (0D) carbons arrayed along the 1D carbon nanofiber is highly desirable for supercapacitors because it presents improved rate capability and high axial electron conductivity. The presence of Fe, Co and N dopants in the carbon matrix also generates pseudocapacitance to further improve specific capacitance. The optimized 1D-HCNB-900 generates a specific capacitance of 370.0 F g−1 at a current density of 2 A g−1, high rate capability and tolerance, and great cyclability.
- This article is part of the themed collection: 2023 Journal of Materials Chemistry A Lunar New Year collection


 Please wait while we load your content...
Please wait while we load your content...