Controlled electropositive catalytic sites on zeolites for achieving high CH3Cl selectivity via electrophilic CH4 chlorination using Cl2†
Abstract
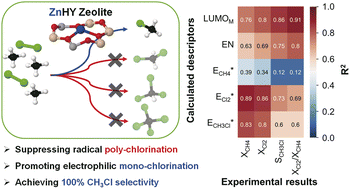
The spontaneous homolytic Cl2 cleavage to two reactive chlorine radicals is useful for activating the C–H bond in CH4 to produce various chlorinated products (CH3Cl, CH2Cl2, CHCl3, and CCl4), but it is noncatalytic and uncontrollable. Herein, zeolites with controlled electropositive Lewis acidic sites originating from transition metal cations with various oxidation states successfully promote the electrophilic CH4 chlorination with suppressing the radical pathway, which achieved nearly 100% selectivity to a desirable monochlorinated product (CH3Cl). The experimental results were clearly interpreted based on the selected catalytic descriptors calculated by the DFT method, in which the calculated Cl2 adsorption energy to the catalytic sites, and LUMO energy levels of transition metal cations showed strong correlations with conversion of reactants and the CH3Cl selectivity. Consequently, Zn2+-incorporated zeolite (ZnHY) achieved nearly 100% CH3Cl selectivity without deactivation. The comprehensive experimental and theoretical investigations offer valuable insights for the design of zeolite-based catalysts promoting electrophilic CH4 chlorination using Cl2.


 Please wait while we load your content...
Please wait while we load your content...