“Uncapped” metal–organic framework (MOF) dispersions driven by O2 plasma towards superior oxygen evolution electrocatalysis†
Abstract
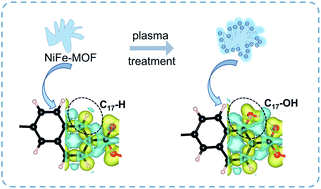
The lack of solution processability of metal–organic frameworks (MOFs) has been a major obstacle hampering their practical applications. At present, a common belief is that the solution processability of MOFs can only be improved by functionalization with foreign stabilizers. In this work, we firstly predicted through density functional theory (DFT) that in case of a slight excess of the oxygen percentage of MOFs (∼15%), the ionization of additional hydroxyl groups on the surface of MOFs can greatly enhance the solution dispersibility of MOFs through electrostatic stabilization. We consequently demonstrated a general protocol for manipulating MOFs' solution processability through O2 plasma treatment (0–40 min), which can continuously adjust their surface charge in aqueous dispersions (zeta potentials: −13.3–−29.4 mV). This has resulted in a class of “uncapped” MOF aqueous colloids. Further size fractionation of these MOFs in aqueous colloids has produced small-sized MOFs with superior electrocatalytic performance towards the oxygen evolution reaction (overpotential of 258 mV at 10 mA cm−2, small Tafel slope of 19 mV dec−1, and strong durability up to 100 h).


 Please wait while we load your content...
Please wait while we load your content...