Open Access Article
Open Access ArticleRuthenium oxychloride supported by manganese oxide for stable oxygen evolution in acidic media†
Yunxing
Zhao‡
a,
Jun
Hu‡
 b,
Chao-Lung
Chiang
b,
Chao-Lung
Chiang
 c,
Ying
Li
a,
Weichuang
Yang
d,
Zhenhai
Yang
d,
Wei-Hsuan
Hung
e,
Yan-Gu
Lin
c,
Zhong
Chen
c,
Ying
Li
a,
Weichuang
Yang
d,
Zhenhai
Yang
d,
Wei-Hsuan
Hung
e,
Yan-Gu
Lin
c,
Zhong
Chen
 f,
Bin
Li
a,
Pingqi
Gao
f,
Bin
Li
a,
Pingqi
Gao
 *a and
Hong
Li
*a and
Hong
Li
 *ghi
*ghi
aSchool of Materials, Sun Yat-sen University, Guangzhou 510275, China. E-mail: gaopq3@mail.sysu.edu.cn
bSchool of Chemical Engineering, Northwest University, Xi'an 710069, China
cScientific Research Division, National Synchrotron Radiation Research Center, Hsinchu 30076, Taiwan
dNingbo Institute of Material Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China
eInstitute of Materials Science and Engineering, National Central University, Taoyuan 320317, Taiwan
fSchool of Materials Science and Engineering, Nanyang Technological University, 639798, Singapore
gSchool of Mechanical and Aerospace Engineering, Nanyang Technological University, 639798, Singapore
hCINTRA, CNRS/NTU/THALES, UMI 3288, Research Techno Plaza, 637553, Singapore
iCentre for Micro-/Nano-electronics (NOVITAS), School of Electrical and Electronic Engineering, Nanyang Technological University, 639798, Singapore. E-mail: ehongli@ntu.edu.sg
First published on 2nd September 2022
Abstract
Despite the recent advances in enhancing the durability and reducing the overpotential of ruthenium (Ru)-based electrocatalysts for acidic oxygen evolution reaction (OER), their stability hardly meets the requirement of practical application. Moreover, a cost-effective strategy to stabilize the highly active but unstable Ru species is desirable. Herein, we report a stable electrocatalyst for acidic OER by dispersing the Ru oxychloride active species into a manganese oxide support (RuOCl@MnOx) to form highly dispersed Ru–O–Mn without the alteration of vibrational modes and bond parameters of the MnO6 group, as suggested by Raman and synchrotron radiation characterization studies. The catalyst is stable for continuous operation over 280 h with an overpotential of 228 mV at 10 mA cm−2 and over 200 h at 100 mA cm−2, among the most stable low-mass-loading Ru-based OER electrocatalysts in acidic media. Complementary theoretical calculations ascribe the excellent stability to its high oxidation potential and low formation/surface energies, consistent with experimental observations. The enhanced activity is attributed to the four-coordinated Ru site that bears a low overpotential determined by the formation of O* from OH*. Our work thus offers a new strategy for synthesizing robust OER electrocatalysts of PEM electrolyzers with superior activity.
Introduction
Hydrogen fuel is regarded as a promising energy carrier to replace conventional fossil fuels for a sustainable energy future.1 A clean and sustainable hydrogen economy can be truly established only when hydrogen is made from water splitting technology driven by renewable energy sources such as wind and solar.2 Proton-exchange membrane (PEM) electrolysis for hydrogen production has demonstrated many exclusive advantages over alkaline water electrolysis, including higher current density, higher purity of pressurized hydrogen, a more compact and portable electrolyzer system, and importantly, much better compatibility with intermittent/discontinuous renewable energy sources.1,3 Nevertheless, large-scale implementation of a PEM electrolyzer has been restricted by its precious catalysts, particularly the lack of a durable, efficient, and cost-effective electrocatalyst for the oxygen evolution reaction (OER) in harsh acidic media. The state-of-the-art OER catalysts are scarce and precious iridium oxide (IrO2) and ruthenium oxide (RuO2), both of which are perched on top of the OER volcano plot due to their high intrinsic activities.4 RuO2 has a lower cost and higher OER activity but poorer stability than IrO2.5 Hence, developing affordable Ru-based catalysts with long-term operation stability is of great significance for the widespread deployment of PEM electrolysis technology.An inherent low oxidation potential of 1.39 V versus RHE (reversible hydrogen electrode) to form soluble ruthenium tetroxide (RuO4) renders RuO2 less stable under OER operating conditions (>1.23 V vs. RHE), leading to a narrow stable operating potential window.6 Besides, the participation of lattice oxygen of RuO2 to evolve O2 during the OER process also accelerates its degeneration due to corrosion.7 Moreover, the calculated free energy change of the rate determining step (RDS) in the four-step OER reaction is very high at the RuO2 surface, signifying a sluggish kinetic process.8 To this end, tremendous efforts have been dedicated to improving both stability and activity of Ru-based OER catalysts. For instance, vacancy engineering,9 structural defect formation,10,11 element doping,12 metal alloying,13 solid solution design,8 the single atom strategy,14 structure engineering of SrRuO3 perovskite/A2Ru2O7 pyrochlore,15,16 crystal plane/phase engineering,17 and novel catalysts such as RuB2,18 have been reported to exhibit good OER performance, with much lower overpotentials and significantly improved stability (up to 24 h) than those of pristine RuO2 (<4 h)12,19 in delivering a benchmark current density of 10 mA cm−2. However, many approaches focus on structural modification of the crystal or crystalline phase, as well as doping or compounding based on the ruthenium oxide itself, which inevitably introduce the RuO2 crystal in the catalyst, leading to oxidative degradation (by forming soluble RuO4) at low anodic potentials under acidic conditions. A method capable of deconstructing the RuO2 crystal and inhibiting the Ru–O–Ru bonds is expected to greatly enhance the stability of Ru-based OER catalysts.
Moreover, the supporting material for catalysts has been proven to play a key role in catalysis, which reduces the possibility of agglomeration and increases the specific surface area, resulting in better dispersion of active catalyst species and more efficient atom utilization.20,21 Besides, the metal–support interaction is often observed for improving interfacial electron transfer,22 tailoring the binding strength,20 or inducing surface species migration (e.g., hydrogen spillover).23 Towards acidic OER, a supporting material can enhance the stability of catalysts in extremely harsh, corrosive, and oxidative environments. Cerium dioxide (CeO2), titanium dioxide (TiO2), chromium dioxide (CrO2), manganese dioxide (MnO2), titanium (Ti), tellurium (Te), and carbon (C) as potential candidates of supporting materials have demonstrated superb acid and oxidation resistance as evidenced by their nanocomposite (e.g., Co3O4/CeO2),24 composite catalyst (e.g., IrO2–TiO2),25 solid solution (e.g., Cr0.6Ru0.4O2),8 and catalyst on supports (e.g., IrO2/MnO2, H-Ti@IrOx, IrRu@Te, and RuIr@CoNC).26–29 Notably, individual gamma (γ)-phase MnO2 as a nonprecious OER catalyst shows stable operation over 8, 000 h at 10 mA cm−2 in an acidic electrolyte (pH of 2), indicating its inherent chemical stability; although the overpotential (428 mV) is much inferior to that of RuO2 (310 mV).30
Herein, a stable Ru-based OER catalyst is rationally constructed by dispersing the Ru oxychloride active species into a solid MnOx supporting material, where Ru is the actual active site, and the doped/chemisorbed Cl facilitates the dispersion of Ru and thus provides abundant active structural defects.31,32 The rational design of the catalyst comprises: (1) the MnOx support with high acid and oxidation resistance helps stabilize the encapsulated Ru species by inhibiting RuO2 formation, (2) the Ru active sites significantly decrease the OER overpotential, which in turn slows down the oxidation of the MnOx support to MnO4− at high potentials, (3) the homogeneous dispersion of the active Ru sites in the MnOx matrix results in a sustained OER operation with high activity at low mass loading, and (4) both OER-active RuO2 and MnOx with strong catalyst–support interactions work synergistically to enhance the overall catalytic performance. The resulting catalyst with a low Ru mass loading of 0.105 mgRu cm−2 (versus 1–3 mgIr cm−2 for commercial OER catalysts used in PEM electrolyzers)1 shows a low overpotential of 228 mV at 10 mA cm−2 and great stability for 280 h at 10 mA cm−2 and 200 h at 100 mA cm−2 in the strongly acidic media (0.5 M sulfuric acid, pH 0.26), outperforming most reported Ru-based OER electrocatalysts.
Results and discussion
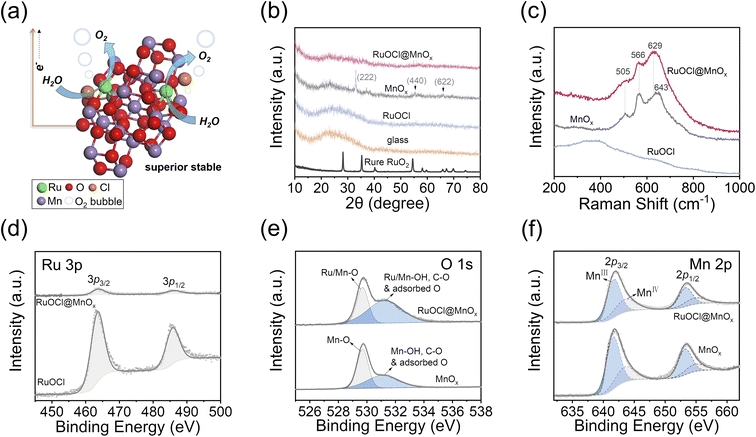
The facile fabrication process of the anode material is illustrated in Fig. S1a.† First, a carbon fiber paper (CFP) substrate was heated to 210 °C, followed by the dropwise addition of a precursor solution containing RuCl3 and Mn(NO3)2 onto the hot CFP surface (see the Experimental section, ESI†). Then, RuOCl@MnOx was obtained after heating the CFP-loaded precursor at 210 °C for 10 min. The MnOx or RuOCl control samples were obtained by dropwise adding individual RuCl3 or Mn(NO3)2 solutions onto the CFP substrate, while RuOCl/MnOx was obtained by dropwise adding RuCl3 solution onto the as-synthesized CFP-loaded MnOx, all of which were made by the same thermal treatment process. The CFP substrate is suitable for fabrication of a membrane electrode assembly in PEM electrolyzers owing to its excellent resistance to acid and oxidation.30,33 Notably, this fabrication method is scalable thanks to the mild synthesis conditions and low cost of MnO2 (10−4 times lower than that of RuO2). The schematic catalyst structure of RuOCl@MnOx is illustrated in Fig. 1a, where the Ru oxychloride (RuOCl) species is dispersed into the MnOx support. The Ru atoms bond to O atoms to form the dominant Ru–O–Mn, and the residue Cl element is doped or chemisorbed in the catalyst after oxidation of the RuCl3 precursor. A defect structure is introduced onto the MnOx support arising from exotic Ru atoms, and the doped/chemisorbed Cl atom further increases the unsaturated coordination defects. The active Ru atoms are encapsulated by abundant Mn and O atoms, thus providing protection to the catalytic site. Such a geometry has several advantages: (1) the similar octahedral coordination of RuO2 and MnO2 as their crystalline phases34 endows RuOCl@MnOx with compatible unit structures and thus suppresses the tendency of phase segregation, (2) the amorphous characteristics arising from the low synthesis temperature increase the density of defect sites, such as grain boundaries and edges, and thus increase the catalytic activity, and (3) the MnOx material with excellent resistance to acid corrosion and oxidation offers a reliable and stable support for the Ru catalyst, thus breaking the aggregation of ruthenium oxide and improving the oxidation potential.The structure analysis by X-ray diffraction (XRD; Fig. 1b and S1b†) indicates the amorphous state of RuOCl@MnOx and RuOCl, as suggested by the lack of well-defined peaks. The XRD peaks of MnOx located at 2θ = 33.1, 55.2, and 66.0° correspond to the (222), (440), and (622) planes of the Mn2O3 structure, respectively.35 The absence of a crystalline peak in RuOCl@MnOx suggests that the added Ru and Cl disturb the crystallinity of the MnOx support. It is worth noting that crystal defects and unsaturated coordination in the RuO2 catalyst are responsible for the highly active OER.10,12,36 The Raman spectra (Fig. 1c) show that the peak positions of RuOCl@MnOx are close to those of MnOx, where 505 cm−1, 566 cm−1 (Mn–O stretching vibration in the basal plane), and 643 cm−1 (symmetric stretching mode of the MnO6 group) correspond to the lattice vibration modes of MnO2.37,38 The RuOCl sample does not show the lattice vibration peaks observed in crystalline RuO2.36 It is therefore unlikely to form crystalline RuO2 from the aqueous RuCl3 precursor by treatment at 210 °C for 10 min, whereas crystalline MnOx can be obtained by pyrolysis of Mn(NO3)2 under these conditions. The elemental composition analyses were conducted by X-ray photoelectron spectroscopy (XPS) for Ru 3p, Cl 2p, Mn 2p, and O 1s states. The full XPS spectrum in Fig. S2a† covers Ru, O and Cl elements in catalysts RuOCl@MnOx and RuOCl, as well as Mn and O elements from the MnOx support. Ru 3p peaks located at 463.8 and 486.2 eV (Fig. 1d) and Ru 3d peaks at 281.3 and 285.5 eV (Fig. S2b†) are assigned to the Ru4+ state.10,39 We note that the intensity of Ru in RuOCl@MnOx is much weaker than that in RuOCl even at the same Ru loading, indicating good dispersion of Ru into the MnOx matrix. Notably, a significant Cl signal is observed in both RuOCl@MnOx and RuOCl (Fig. S2c†) at 198.1 eV and 199.8 eV, corresponding to Cl 2p3/2 and Cl 2p1/2 of Cl−.40 The Cl could not be completely removed despite the thorough cleaning of the prepared sample, suggesting that the Cl element has been doped or chemically adsorbed in the catalyst. In addition, the O 1s spectra (Fig. 1e) can be deconvoluted as the lattice oxygen (529.7 eV) and hydroxyl groups/C–O/adsorbed oxygen on the surface (531.2 eV).10,11 The Mn 2p3/2 signals located at 641.7 eV and 643.6 eV (Fig. 1f) in RuOCl@MnOx and MnOx are assigned to Mn3+ and Mn4+ states,41 respectively, with a ratio of ∼1.8 (Mn3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+) in RuOCl@MnOx. Moreover, the Ru
Mn4+) in RuOCl@MnOx. Moreover, the Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl
Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn ratio (1
Mn ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.5
15.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8) in RuOCl@MnOx was obtained by semi-quantitative analysis of XPS (Fig. S2d†), which reveals that each Ru catalytic site is surrounded by 8 Mn atoms, suggesting its good dispersion. In contrast, the Cl content is significantly less in RuOCl (Ru
7.8) in RuOCl@MnOx was obtained by semi-quantitative analysis of XPS (Fig. S2d†), which reveals that each Ru catalytic site is surrounded by 8 Mn atoms, suggesting its good dispersion. In contrast, the Cl content is significantly less in RuOCl (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl
Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O = 1
O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1), suggesting that some Cl would be doped or chemisorbed into the MnOx. And the Mn
2.1), suggesting that some Cl would be doped or chemisorbed into the MnOx. And the Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O (1
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8) ratio in MnOx implies a mixture of polycrystalline Mn2O3 and amorphous MnO2 in the MnOx support, consistent with XRD analysis. The K-edge XANES spectra are analyzed to further confirm the oxidation states of Ru and Mn, by fitting the Ru/Mn oxidation states as a function of the Ru/Mn K-edge energy shifts (Fig. S3†), and the average valence states of Ru and Mn are Ru3.9+ and Mn2.8+, respectively.
1.8) ratio in MnOx implies a mixture of polycrystalline Mn2O3 and amorphous MnO2 in the MnOx support, consistent with XRD analysis. The K-edge XANES spectra are analyzed to further confirm the oxidation states of Ru and Mn, by fitting the Ru/Mn oxidation states as a function of the Ru/Mn K-edge energy shifts (Fig. S3†), and the average valence states of Ru and Mn are Ru3.9+ and Mn2.8+, respectively.
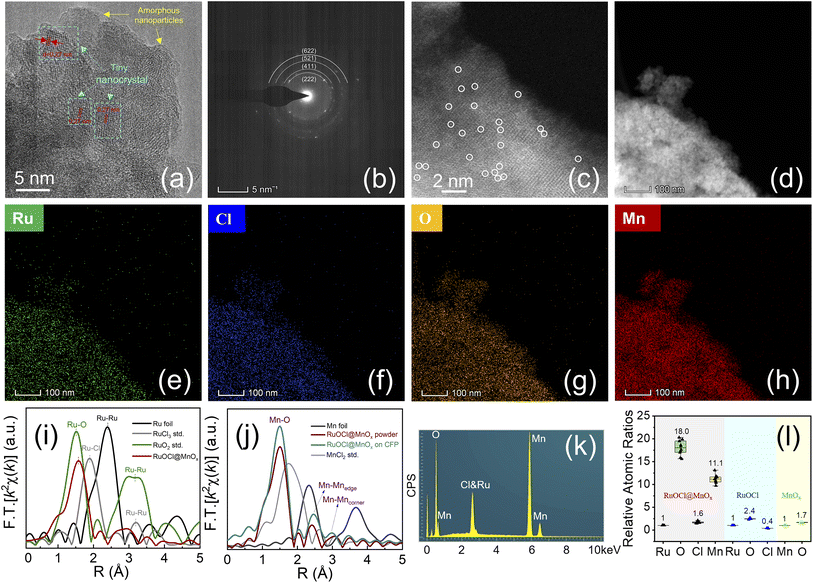
The high-resolution transmission electron microscopy (HRTEM) image in Fig. 2a shows that the RuOCl@MnOx is composed of amorphous nanoparticles with tiny nanocrystals, where a d-spacing of 0.27 nm corresponds to the (222) plane of Mn2O3.42 Besides, the selected area electron diffraction (SAED, Fig. 2b) patterns show faint diffraction rings corresponding to the (222), (411), (521) and (622) planes of Mn2O3, further illustrating the polycrystalline and amorphous composition of RuOCl@MnOx.43–45 The absence of RuO2 nanocrystals indicates great dispersion of Ru in the MnOx support. The image taken by aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM, Fig. 2c) shows that some individual metal atoms (i.e., Ru and Mn) are well anchored on the surface of the MnOx support as distinguishable bright spots marked with circles. The element distributions in RuOCl@MnOx were determined by scanning TEM energy dispersive X-ray spectroscopy (STEM-EDS), and the images show that all elements including Ru, Cl, O, and Mn exhibit a uniform distribution (Fig. 2d–h) in the catalyst. The Fourier transforms (FTs) of the Ru K-edge k2χ(k) spectra (Fig. 2i) show that the Ru–O peak shifts to 1.57 Å of RuOCl@MnOx from 1.50 Å of RuO2, which is attributed to the change in the local coordination environment induced by the dominant Ru–O–Mn bond in RuOCl@MnOx.46,47 The FTs of the Mn K-edge k2χ(k) spectra (Fig. 2j), where 1.5, 2.4 and 3.0 Å match the Mn–O bond, the edge-sharing Mn–Mnedge, and the corner-sharing Mn–Mncorner in the MnO6 octahedra, respectively,48 suggest that the MnO6 octahedral framework in the MnOx support is maintained despite the introduction of external elements, which is consistent with the Raman measurement results. In contrast to the Ru–Cl (1.90 Å) and Mn–Cl (1.75 Å) coordination in the first shells of Ru and Mn central atoms in RuCl3 and MnCl2, respectively, the peaks at 1.57 Å and 1.5 Å in RuOCl@MnOx (Fig. 2i and j) correspond to the Ru–O and Mn–O coordination in the first shells of Ru and Mn sites, respectively. This suggests that the Ru–Cl coordination in RuCl3 is replaced by Ru–O during the thermal treatment, while the Mn–O coordination is generated during the pyrolysis of Mn(NO3)2. A large number of Cl atoms in the non-first coordination shell of Ru or Mn centers may physically or chemically interact with O atoms, resulting in the adsorption or doping state, which is not detected in the K-edge FT-EXAFS spectra.49,50 In addition, the electron energy loss spectroscopy (EELS) pattern (Fig. S4†) shows that only Mn and O signals appear in RuOCl@MnOx, in contrast to the obvious Ru and Cl peaks in RuOCl. The absence of Ru and Cl peaks due to the encapsulation by the manganese oxide support suggests that RuOCl has been uniformly dispersed in the MnOx matrix rather than segregated on the surface. The SEM images (Fig. S5†) show that both RuOCl@MnOx and MnOx are densely deposited on the CFP surface; however, the coverage of RuOCl is poor. SEM-EDS analysis was also employed to quantify the elemental content (Fig. S6†), which shows the uniform distribution of Ru, Cl, O, and Mn on the deposited membrane. The Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl
Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn (1
Mn (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6
1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.0
18.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.1) ratios in RuOCl@MnOx (see Fig. 2k for the spectrum) and the Ru
11.1) ratios in RuOCl@MnOx (see Fig. 2k for the spectrum) and the Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl
Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O (1
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4) ratios in RuOCl (Fig. 2l) obtained from EDS indicate that the proportion of Cl is higher by EDS than that by XPS, suggesting that more Cl is doped or chemisorbed beneath the surface. Furthermore, the Mn
2.4) ratios in RuOCl (Fig. 2l) obtained from EDS indicate that the proportion of Cl is higher by EDS than that by XPS, suggesting that more Cl is doped or chemisorbed beneath the surface. Furthermore, the Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O (1
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7) ratio measured by EDS is close to that obtained by XPS, suggesting a mixture of polycrystalline Mn2O3 and amorphous MnO2. The loading mass of Ru in RuOCl@MnOx is 0.112 mg cm−2 (calculated as MW = 207.43 g mol−1 for RuCl3·H2O) or 0.105 mg cm−2 [based on inductively coupled plasma optical emission spectrometry (ICP-OES)], corresponding to 0.15 mg or 0.14 mg pristine RuO2, respectively. ICP-OES (Fig. S7†) also gives the Ru
1.7) ratio measured by EDS is close to that obtained by XPS, suggesting a mixture of polycrystalline Mn2O3 and amorphous MnO2. The loading mass of Ru in RuOCl@MnOx is 0.112 mg cm−2 (calculated as MW = 207.43 g mol−1 for RuCl3·H2O) or 0.105 mg cm−2 [based on inductively coupled plasma optical emission spectrometry (ICP-OES)], corresponding to 0.15 mg or 0.14 mg pristine RuO2, respectively. ICP-OES (Fig. S7†) also gives the Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn (1
Mn (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.6) ratio in RuOCl@MnOx, which is close to those estimated from EDS or XPS analysis (Table S1†).
9.6) ratio in RuOCl@MnOx, which is close to those estimated from EDS or XPS analysis (Table S1†).
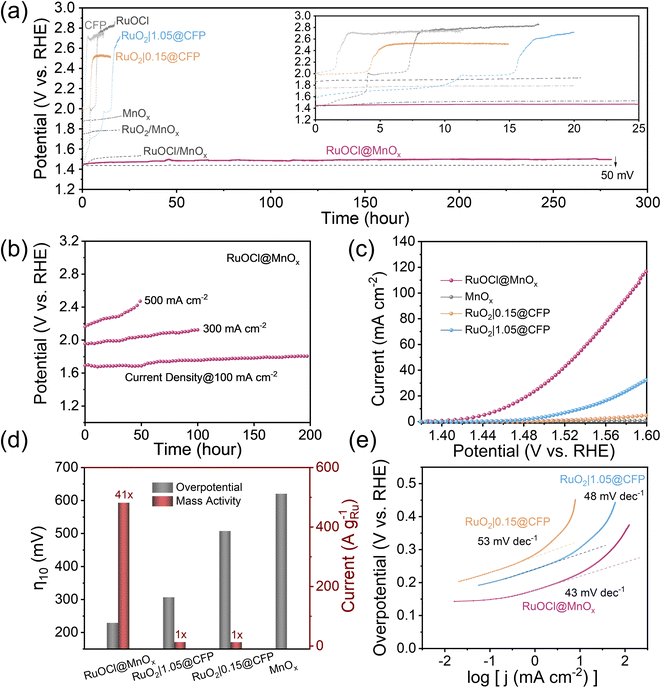
Electrochemical tests were carried out on a three-electrode setup with a catalyst-coated CFP as the working electrode (Fig. S8†) to evaluate the stability and activity of the catalyst. The stability test was recorded as shown in Fig. 3a, which exhibits the potential change at a constant OER current density of 10 mA cm−2. The RuOCl@MnOx catalyst displays stable operation up to 280 h with only a 50 mV overpotential increase, corresponding to a degradation rate of 0.18 mV h−1. Notably, most of the degradation (46%) occurs in the first 25 h (the inset of Fig. 3a). This superior stability outperforms most Ru-based OER electrocatalyst operating in acidic media (see Table S2† for comparison), while being comparable to other support-loaded Ru/Ir catalysts.27–29 In contrast, both pristine RuO2 (denoted as RuO2|x@CFP, with x representing the RuO2 mass loading by dispersing RuO2/Nafion/water/ethanol ink on CFP, see the Experimental section in the ESI†) and RuOCl exhibit rapid activity decay. Specifically, RuO2|0.15@CFP and RuO2|1.05@CFP (with 1 and 7 times as much Ru mass loading as that in RuOCl@MnOx, respectively) completely deactivate within 0.05 h and 10 h, respectively, consistent with previous reports of rapid RuO2 degradation.8,19 Meanwhile, the potential of RuOCl increases from 1.44 to 2 V within 4 h, corresponding to a degradation rate of 140 mV h−1. Moreover, the relatively steady OER potential of MnOx with a minor increase from 1.86 V to 1.93 V over 20 h discloses its high antioxidant capability but inferior OER activity. These comparisons suggest that Ru is the active site, and MnOx acts as the supporting material, endowing RuOCl@MnOx with superior stability and activity. Additionally, we note that the potentials of RuOCl/MnOx and RuO2/MnOx (prepared by dropping RuO2/Nafion/water/ethanol dispersion ink onto the surface of the as-synthesized CFP-loaded MnOx) undergo a much slower decrease to 1.54 V and 1.79 V in 30 h and 20 h, respectively, compared to the rapid deactivation of RuOCl (4 h) and RuO2|0.15@CFP (0.05 h), signifying that MnOx also stabilizes the catalyst even by simple loading on its surface. We hypothesize that the enhanced durability of RuOCl/MnOx and RuO2/MnOx is ascribed to the valence state and structural transformation of MnOx during the OER process that will be detailed later. However, the Ru content of RuO2/MnOx is reduced due to the degradation of surface RuO2, similar to that of pure RuO2 in the stability test. The inhomogeneous structure of Ru, which is physiochemically adsorbed at the RuO2 and MnOx interface or encapsulated in structurally transformed MnOx, makes RuO2/MnOx less active than RuOCl@MnOx. Steady OER operation at high current densities accelerates the oxidative failure of the catalyst, and thus it is an important consideration in practical applications. We therefore tested the stability at higher current densities of 100 mA cm−2, 300 mA cm−2 and 500 mA cm−2 as shown in Fig. 3b. After 200 h continuous operation at 100 mA cm−2, the overpotential increment is 115 mV, corresponding to a degradation rate of 0.6 mV h−1. The degradation accelerates at 300 mA cm−2 (164 mV degradation in 100 h) and 500 mA cm−2 (346 mV degradation in 50 h).
Next, we evaluated the activity of the catalyst RuOCl@MnOx from the linear scanning voltammetry (LSV) curves (Fig. 3c). RuOCl@MnOx exhibits excellent activity with an onset potential (the potential at a current density of 1 mA cm−2) of 1.41 V and an overpotential of only 228 mV at 10 mA cm−2. In contrast, the onset potentials of RuO2|0.15@CFP and RuO2|1.05@CFP are much higher, reaching 1.52 V and 1.47 V, respectively. The overpotential of RuO2|1.05@CFP is 306 mV at a current density of 10 mA cm−2 (Fig. 3d). RuO2|1.05@CFP and RuO2|0.15@CFP show small current densities of 32 and 5 mA cm−2 (vs. 118 mA cm−2 of RuOCl@MnOx) at 1.6 V. Moreover, a high onset potential of 1.76 V and a high overpotential of 620 mV at 10 mA cm−2 for MnOx also indicate that the active site is not derived from the MnOx support. In addition, RuOCl suffered a rapid decay in the potential scan test (Fig. S9†). It is noted that RuO2|0.15@CFP with the same Ru mass loading as that of RuOCl@MnOx (calculated as MW = 207.43 g mol−1 for RuCl3·H2O) has a much higher overpotential than 306 mV when driving a current density of 10 mA cm−2, consistent with the previous reports.36 Upon gradually increasing the Ru loading (Fig. S10a†), the overpotential of RuO2|1.05@CFP reaches 306 mV, which is close to that of RuO2 loaded on a glassy carbon electrode (∼0.275 mg cm−2).12 Such inferior performance is attributed to the fact that the loaded RuO2 nanoparticles (TEM image in Fig. S10b†) are partially trapped in the interfibrillar voids of CFP and therefore cannot participate efficiently in the catalytic reaction. The mass activity (Fig. S11†) of RuOCl@MnOx is as high as 481 A gRu−1 at η = 300 mV, which is 41 times higher than that of RuO2|1.05@CFP or RuO2|0.15@CFP (Fig. 3d). Although the mass activity of catalysts with active sites uniformly dispersed inside the support is relatively low compared to those with active sites dispersed on the support surface,27–29 RuOCl@MnOx is still one of the most superior low-mass-loading Ru- and Ir-based electrocatalysts (see Table S2† for details). Notably, the Tafel curve (Fig. 3e) of RuOCl@MnOx (43 mV dec−1) is smaller than those of RuO2|1.05@CFP (48 mV dec−1) and RuO2|0.15@CFP (53 mV dec−1), indicating faster OER kinetics.
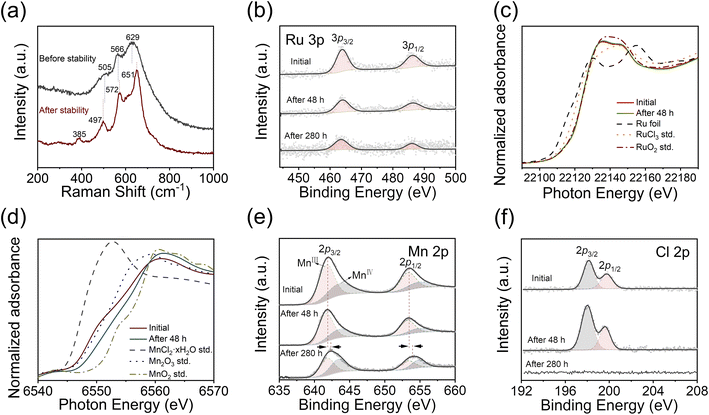
We examined the structural stability of the catalyst after 280 h stability testing. Compared with the initially rough and dense membranes (Fig. S12a–c†), the catalyst appears to resemble the nanosheet structure of MnO2 after stability testing (Fig. S12d–f†). The Raman peaks are slightly shifted to 497 cm−1, 572 cm−1, and 651 cm−1 as shown in Fig. 4a, which may have originated from the changes in the valence state and bond length.51,52 The newly appeared peak located at 385 cm−1 corresponds to the bending mode of Mn–O–Mn.53 However, the XRD pattern (Fig. S13†) does not show any crystalline MnO2 peak, which suggests a predominantly amorphous phase of MnO2. It is still possible to observe some tiny nanocrystals with a lattice spacing of 0.36 nm by HRTEM (Fig. S14†), which corresponds to the (002) plane of δ-MnO2,54 suggesting the transformation of the MnOx support during the catalysis process. The XPS and normalized Ru K-edge XANES spectra of RuOCl@MnOx after 48 h testing (Fig. 4b and c) show no significant degradation of the Ru active site during the early stages of long-term catalysis. The Mn K-edge XANES spectra shift from an energy close to Mn2O3 (Mn3+ reference) towards that of MnO2 (Mn4+ reference) after 48 h testing, while a significant positive shift of the Mn 2p peak is observed by XPS after 280 h (Fig. 4d and e). Concurrently, the Mn3+/Mn4+ ratio gradually decreases from 1.8 (initially) to 1.1 (after 280 h), further indicating an increase in MnO2, which is consistent with the morphological and Raman characterization studies. Doped/chemisorbed Cl (Fig. 4f) is closely related to the degradation of the catalyst. With the Cl peak disappearing after the 280 h test, apparent degradation occurs thereafter. In addition, the O 1s peak (Fig. S15†) of lattice oxygen shifts slightly after the 280 h test, which could be attributed to the composition, valence state, and microstructure of the redeposited MnO2 nanosheet.55,56 The weights of Mn and Ru ions dissolved in solution after 280 h stability testing are 0.08 mg and 0.07 mg, respectively, according to ICP measurement (Fig. S7†). Other dissolved Mn3+, however, is redeposited on the surface in the form of MnO2 nanosheets (Fig. S12 and S14†), which is consistent with a previous report.30 Notably, the high activity of the catalyst is not significantly affected during the redeposition process of MnO2 nanosheets, suggesting that the vertical structure of the nanosheets provides sufficient space for the transport of the proton and hydrogen molecule. The faradaic efficiency (electric charges involved) of the Mn/Ru ionic reactions is negligible compared to those of the OER or HER (ESI Note 1†). Moreover, sheet-like structures with morphological variations depending on the applied potentials are also observed after stability tests at high current densities (Fig. S16†), and the flakes are dominated by the amorphous phase as shown by HRTEM (Fig. S17†). The Raman peaks (Fig. S18†) exhibit slight shifts, accompanied by the appearance of peaks located at 385 cm−1 (bending vibration of Mn–O–Mn) and 720 cm−1 (stretching vibration of Mn–O–Mn).53,57 Additionally, the Ru 3p peaks are significantly weakened, reflecting the imminent depletion of the active species on the catalyst surface and thus the impending rapid decay of activity, approaching the end of the stability test; furthermore, both the Mn 2p and O 1s peaks show similar positive shifts (Fig. S19†). The Mn and Ru ions undergo more rapid dissolution at high anodic potentials as revealed by ICP-OES characterization (Fig. S7b†).
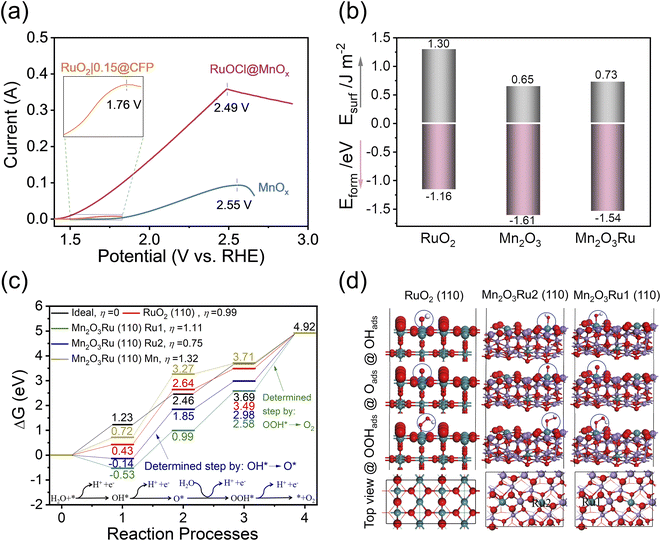
MnO2 and RuO2 corrode via the following reactions, MnO2 + 2H2O → MnO4− + 4H+ + 3e− (E0 = 1.70 V vs. RHE)58 and RuO2 + 2H2O → RuO4(aq) + 4H+ + 4e− (E0 = 1.39 V vs. RHE).6 For the durability test, the MnOx catalyst support remains stable for at least 20 h in the range of 1.86 to 1.93 V (Fig. 3a). Our calculated Pourbaix diagram (Fig. S20†) of the 89–11% Mn–Ru system in aqueous solution also demonstrates a higher oxidation potential of MnO2 than RuO2. We also performed a slow scan (0.1 mV s−1) LSV experiment extended to high anodic potentials (Fig. 5a), and the results show that the OER dominates prior to catalyst degradation, and the significant degradation occurs at the clear inflection points of OER current. For RuO2|0.15@CFP, RuOCl@MnOx, and MnOx, the potentials at the inflection points are 1.76 V, 2.49 V, and 2.55 V, respectively. One can see that the MnOx support greatly improves the oxidation potential of the RuOCl@MnOx catalyst. It is noteworthy that the elevated oxidation potential is crucial for enhancing the stability. The dissolution of the RuO2 lattice, which is triggered by the loss of lattice/surface oxygen accompanied by the sharing of OER intermediates,7,59 depends on the applied potential, and it becomes more severe when the potential is much higher than the theoretical redox potential (1.39 VRHE, pH 0)6. The dispersion of Ru into the MnOx support results in a high oxidation potential of the catalyst and therefore long-term stability even at high current density.
In view of the predominantly amorphous characteristic of the catalyst, the catalytic reaction may involve the participation of lattice/surface oxygen.60 Distinct from the Ru–O–Ru bonding in conventional RuO2, the Ru–O–Mn bonding dominates here as a Ru atom can be surrounded by nearly ten times as many Mn atoms according to ICP-OES tests (Table S1†). Due to the difference in bond length, electronegativity, and bond strength between Mn–O and Ru–O,34,61 the RuOCl@MnOx catalyst differs from RuO2 in terms of recovery of the catalyst structure such as oxygen vacancies. The oxygen vacancies involving lattice/surface oxygen accelerate the dissolution of RuO2,7 whereas the enhanced stability of the Ru catalyst is attributed to the presence of MnOx that could promote the structure recovery and thus reduce the catalyst degradation. Therefore, we calculated the bulk formation energy and surface energy for the three models of RuO2, Mn2O3, and Mn2O3Ru (see Fig. S21† for the simulation models and optimal bond parameters), since the formation energy and surface energy are key parameters to assess the structural stability. It is worth noting that Mn2O3 is appropriate as the simulation model for the MnOx support based on the experimental XRD, HRTEM, XPS and Mn K-edge XANES analyses, which show that Mn3+ is dominant. Moreover, the doped/chemisorbed Cl is not included in the model because it is not the first shell coordination atom for Ru or Mn central sites as suggested by the K-edge FT-EXAFS spectra (Fig. 2i and j), and it is not supposed to be directly engaged in the OER catalytic process. As depicted in Fig. 5b, the formation energies of RuO2, Mn2O3 and Mn2O3Ru are −1.16 eV, −1.61 eV and −1.54 eV, while the surface energies are 1.30 eV, 0.65 eV and 0.73 eV, respectively. Notably, both the bulk formation energy and surface energy of Mn2O3 are lower than those of RuO2, suggesting that Mn2O3 is more stable than RuO2. Moreover, it suggests that even when 9% Mn is substituted by Ru (i.e., Mn2O3Ru), the energy is still lower than that of RuO2, which signifies the greatly enhanced structural stability of Mn2O3Ru than pure RuO2.
The calculated Gibbs free energy (Fig. 5c) for the OER process on different surfaces are displayed to evaluate the intrinsic activity. Here, we constructed simulation models of Mn2O3Ru with 9% Ru close to the experimentally measured value, as well as RuO2 as a reference, as depicted in Fig. 5d. Ru serving as a surface catalysis center has an unsaturated coordination structure (the coordination number of Ru is determined by using the Calculate Bonds tool, Experimental section), where Ru in RuO2 and Ru1 in Mn2O3Ru have a five-coordinated structure, and Ru2 in Mn2O3Ru has a four-coordinated structure, and these unsaturated sites are analogous to the lattice/surface oxygen vacancies for catalysis (Fig. S22†). The adsorbate evolution mechanism (AEM) is mainly considered here because a recent report suggests that the lattice oxygen evolution makes a negligible contribution to the overall OER activity of RuOx in acidic electrolyte.59 The energy of O2 in an ideal catalysis process is estimated to be ΔGO2 = 4 × 1.23 = 4.92 eV since it is a four-electron-transfer process.62 Comparing the Gibbs free energy on different surfaces, the reaction process of the Ru2 site on the Mn2O3Ru (110) surface is closer to the ideal catalysis process than that of pure RuO2 and the Ru1 site on the Mn2O3Ru (110) surface, since the theoretical overpotentials for the Ru2 site on Mn2O3Ru (110), RuO2, Ru1 site on Mn2O3Ru (110), and Mn site on Mn2O3Ru (110) surfaces are 0.75, 0.99, 1.11, and 1.32 V, respectively. The formation of O* from OH* serves as the rate determining step (RDS) for the four-coordinated Ru2 site that bears the smallest overpotential, although it is still higher than the experimental overpotential because the effects of solution and temperature are not taken into account in the modelling. In contrast, the reaction on the five-coordinated Ru1 site is limited by the formation of O2 from OOH*, accompanied by a larger overpotential than that of RuO2. The large overpotential of the Mn site also illustrates its role as a supporting material. In addition, a very large overpotential is observed if Cl is considered as the first-shell coordination atom for Ru and Mn sites (Fig. S23†), which further suggests that Cl does not bond directly to the metal atoms, in agreement with the FT-EXAFS results. Nevertheless, Cl can indirectly enhance the catalytic performance of Ru by enriching the defect sites and facilitating the dispersion of Ru. Overall, the enhanced activity is related to the four-coordinated unsaturated ruthenium structure in the RuOCl@MnOx catalyst.
Conclusions
We have developed a stabilization strategy by dispersing catalytically active RuOCl species into a low-cost MnOx support to obtain a cost-effective, active, and stable OER electrocatalyst in an acidic electrolyte. The catalyst delivers 10 mA cm−2 at an overpotential of 228 mV, a mass activity of 481 A gRu−1 at an overpotential of 300 mV, and a Tafel slope of 43 mV dec−1. More importantly, it exhibits excellent stability for 280 hours at 10 mA cm−2, far exceeding those of pristine RuO2 or support-free RuOCl catalysts. Stable operation over 200, 100, and 50 hours at 100, 300, and 500 mA cm−2, respectively, has been demonstrated. Combining synchrotron radiation and other characterization studies, the outstanding stability is attributed to the synergistic effect of the catalytic species and the support, where the MnOx support with high resistance to acid and oxidation increases the oxidation potential and slows down the oxidative corrosion of Ru, while the Ru active sites allow the OER to occur at low potentials that in turn suppresses the corrosion of the MnOx support. Our complementary theoretical investigations disclose that the dispersion of RuOCl into the MnOx support increases the oxidation potential and lowers the bulk formation and surface energies. As a result, the durability of the catalyst is drastically enhanced. Notably, the four-coordinated Ru site in the catalyst bears a lower overpotential and thus higher activity than that pure RuO2 catalyst. Our work offers a new strategy for making stable and active OER catalysts with low Ru loading for PEM electrolyzers.Author contributions
Y. Z., P. G., and H. L. designed the studies and wrote the manuscript; Y. Z. carried out the experiments; J. H. carried out DFT calculations; C. C., W. H., and Y. L., performed the XAS experiments and data analysis. Y. L., W. Y., Z. Y., Z. C., and B. L., provided helpful assistance to this article. All authors discussed the results and commented on the manuscript.Conflicts of interest
H. Li is the inventor on a patent related to this work, filed by Nanyang Technological University, Singapore (application no. 2022-228-01-SG PRV). The authors declare no other competing interests.Acknowledgements
The work was partially supported by A*STAR Science & Engineering Research Council AME IRG funding (A1983c0029) and Nanyang Technological University via an ACE grant (NTU-ACE2021-02). H. J. is thankful for financial support from the National Natural Science Foundation of China (Grant No. 21676216). The theoretical work was carried out at the Shanxi Supercomputing Center of China, and the calculations were performed on a TianHe-2.Notes and references
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- S. E. Hosseini and M. A. Wahid, Int. J. Energy Res., 2020, 44, 4110–4131 CrossRef.
- Y. Zhao, M. T. Tang, S. Wu, J. Geng, Z. Han, K. Chan, P. Gao and H. Li, J. Catal., 2020, 382, 320–328 CrossRef CAS.
- I. C. Man, H. Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- T. Weber, T. Ortmann, D. Escalera-López, M. J. Abb, B. Mogwitz, S. Cherevko, M. Rohnke and H. Over, ChemCatChem, 2020, 12, 855–866 CrossRef CAS.
- C. Roy, R. R. Rao, K. A. Stoerzinger, J. Hwang, J. Rossmeisl, I. Chorkendorff, Y. Shao-Horn and I. E. Stephens, ACS Energy Lett., 2018, 3, 2045–2051 CrossRef CAS.
- K. Klyukin, A. Zagalskaya and V. Alexandrov, J. Phys. Chem. C, 2019, 123, 22151–22157 CrossRef CAS.
- Y. Lin, Z. Tian, L. Zhang, J. Ma, Z. Jiang, B. J. Deibert, R. Ge and L. Chen, Nat. Commun., 2019, 10, 162 CrossRef PubMed.
- Y. Tian, S. Wang, E. Velasco, Y. Yang, L. Cao, L. Zhang, X. Li, Y. Lin, Q. Zhang and L. Chen, Iscience, 2020, 23, 100756 CrossRef CAS PubMed.
- Y. Li, Y. Wang, J. Lu, B. Yang, X. San and Z.-S. Wu, Nano Energy, 2020, 78, 105185 CrossRef CAS.
- Z. L. Zhao, Q. Wang, X. Huang, Q. Feng, S. Gu, Z. Zhang, H. Xu, L. Zeng, M. Gu and H. Li, Energy Environ. Sci., 2020, 13, 5143–5151 RSC.
- J. Su, R. Ge, K. Jiang, Y. Dong, F. Hao, Z. Tian, G. Chen and L. Chen, Adv. Mater., 2018, 30, 1801351 CrossRef.
- X. Cui, P. Ren, C. Ma, J. Zhao, R. Chen, S. Chen, N. P. Rajan, H. Li, L. Yu and Z. Tian, Adv. Mater., 2020, 32, 1908126 CrossRef CAS PubMed.
- L. Cao, Q. Luo, J. Chen, L. Wang, Y. Lin, H. Wang, X. Liu, X. Shen, W. Zhang and W. Liu, Nat. Commun., 2019, 10, 4849 CrossRef PubMed.
- M. Retuerto, L. Pascual, F. Calle-Vallejo, P. Ferrer, D. Gianolio, A. G. Pereira, Á. García, J. Torrero, M. T. Fernández-Díaz and P. Bencok, Nat. Commun., 2019, 10, 2041 CrossRef PubMed.
- M. A. Hubert, A. M. Patel, A. Gallo, Y. Liu, E. Valle, M. Ben-Naim, J. Sanchez, D. Sokaras, R. Sinclair and J. K. Nørskov, ACS Catal., 2020, 10, 12182–12196 CrossRef CAS.
- K. A. Stoerzinger, O. Diaz-Morales, M. Kolb, R. R. Rao, R. Frydendal, L. Qiao, X. R. Wang, N. B. Halck, J. Rossmeisl and H. A. Hansen, ACS Energy Lett., 2017, 2, 876–881 CrossRef CAS.
- D. Chen, T. Liu, P. Wang, J. Zhao, C. Zhang, R. Cheng, W. Li, P. Ji, Z. Pu and S. Mu, ACS Energy Lett., 2020, 5, 2909–2915 CrossRef CAS.
- X. Miao, L. Zhang, L. Wu, Z. Hu, L. Shi and S. Zhou, Nat. Commun., 2019, 10, 3809 CrossRef PubMed.
- K. L. Zhou, Z. Wang, C. B. Han, X. Ke, C. Wang, Y. Jin, Q. Zhang, J. Liu, H. Wang and H. Yan, Nat. Commun., 2021, 12, 3783 CrossRef CAS PubMed.
- A. H. Motagamwala, R. Almallahi, J. Wortman, V. O. Igenegbai and S. Linic, Science, 2021, 373, 217–222 CrossRef CAS PubMed.
- K.-Q. Jing, Y.-Q. Fu, Z.-N. Chen, T. Zhang, J. Sun, Z.-N. Xu and G.-C. Guo, ACS Appl. Mater. Interfaces, 2021, 13, 24856–24864 CrossRef CAS.
- J. Li, J. Hu, M. Zhang, W. Gou, S. Zhang, Z. Chen, Y. Qu and Y. Ma, Nat. Commun., 2021, 12, 3502 CrossRef CAS PubMed.
- J. Huang, H. Sheng, R. D. Ross, J. Han, X. Wang, B. Song and S. Jin, Nat. Commun., 2021, 12, 3036 CrossRef CAS PubMed.
- J. Cheng, J. Yang, S. Kitano, G. Juhasz, M. Higashi, M. Sadakiyo, K. Kato, S. Yoshioka, T. Sugiyama and M. Yamauchi, ACS Catal., 2019, 9, 6974–6986 CrossRef CAS.
- Z. Wang, W. Gao, Q. Xu, X. Ren, S. Xu, S. Zhu, X. Niu, X. Li, R. Zhao and Y. Han, ChemElectroChem, 2021, 8, 418–424 CrossRef CAS.
- Z. Yu, J. Xu, Y. Li, B. Wei, N. Zhang, Y. Li, O. Bondarchuk, H. Miao, A. Araujo, Z. Wang, J. L. Faria, Y. Liu and L. Liu, J. Mater. Chem. A, 2020, 8, 24743–24751 RSC.
- J. Xu, Z. Lian, B. Wei, Y. Li, O. Bondarchuk, N. Zhang, Z. Yu, A. Araujo, I. Amorim, Z. Wang, B. Li and L. Liu, ACS Catal., 2020, 10, 3571–3579 CrossRef CAS.
- J. Xu, J. Li, Z. Lian, A. Araujo, Y. Li, B. Wei, Z. Yu, O. Bondarchuk, I. Amorim, V. Tileli, B. Li and L. Liu, ACS Catal., 2021, 11, 3402–3413 CrossRef CAS.
- A. Li, H. Ooka, N. Bonnet, T. Hayashi, Y. Sun, Q. Jiang, C. Li, H. Han and R. Nakamura, Angew. Chem., 2019, 131, 5108–5112 CrossRef.
- V. Giulimondi, S. K. Kaiser, M. Agrachev, F. Krumeich, A. H. Clark, S. Mitchell, G. Jeschke and J. Pérez-Ramírez, J. Mater. Chem. A, 2022, 10, 5953–5961 RSC.
- S. K. Kaiser, R. Lin, F. Krumeich, O. V. Safonova and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2019, 58, 12297–12304 CrossRef CAS PubMed.
- Y. Zhao, J. Hwang, M. T. Tang, H. Chun, X. Wang, H. Zhao, K. Chan, B. Han, P. Gao and H. Li, J. Power Sources, 2020, 456, 227998 CrossRef CAS.
- B. Kang, X. Jin, S. M. Oh, S. B. Patil, M. G. Kim, S. H. Kim and S.-J. Hwang, Appl. Catal., B, 2018, 236, 107–116 CrossRef CAS.
- H. Yan, Q. Shen, Y. Sun, S. Zhao, R. Lu, M. Gong, Y. Liu, X. Zhou, X. Jin and X. Feng, ACS Catal., 2021, 11, 6371–6383 CrossRef CAS.
- R. Ge, L. Li, J. Su, Y. Lin, Z. Tian and L. Chen, Adv. Energy Mater., 2019, 9, 1901313 CrossRef.
- C. Xiong, T. Li, A. Dang, T. Zhao, H. Li and H. Lv, J. Power Sources, 2016, 306, 602–610 CrossRef CAS.
- G. Nie, X. Lu, M. Chi, Y. Zhu, Z. Yang, N. Song and C. Wang, Electrochim. Acta, 2017, 231, 36–43 CrossRef CAS.
- R. Mane, S. Patil, M. Shirai, S. Rayalu and C. Rode, Appl. Catal., B, 2017, 204, 134–146 CrossRef CAS.
- L. Hao, L. Kang, H. Huang, L. Ye, K. Han, S. Yang, H. Yu, M. Batmunkh, Y. Zhang and T. Ma, Adv. Mater., 2019, 31, 1900546 CrossRef PubMed.
- Y. Huang, Y. Li, Z. Hu, G. Wei, J. Guo and J. Liu, J. Mater. Chem. A, 2013, 1, 9809–9813 RSC.
- D. Zhang, J. Cao, X. Zhang, N. Insin, S. Wang, J. Han, Y. Zhao, J. Qin and Y. Huang, Adv. Funct. Mater., 2021, 31, 2009412 CrossRef CAS.
- Y. Ma, Y. Ma, T. Diemant, K. Cao, X. Liu, U. Kaiser, R. J. Behm, A. Varzi and S. Passerini, Adv. Energy Mater., 2021, 11, 2100962 CrossRef CAS.
- E. Moharreri, W. A. Hines, S. Biswas, D. M. Perry, J. He, D. Murray-Simmons and S. L. Suib, Chem. Mater., 2018, 30, 1164–1177 CrossRef CAS.
- D. Jing, D. Chen, G. Fan, Q. Zhang, J. Xu, S. Gou, H. Li and F. Nie, Cryst. Growth Des., 2016, 16, 6849–6857 CrossRef CAS.
- G. Li, H. Jang, S. Liu, Z. Li, M. G. Kim, Q. Qin, X. Liu and J. Cho, Nat. Commun., 2022, 13, 1270 CrossRef CAS PubMed.
- S. Hao, H. Sheng, M. Liu, J. Huang, G. Zheng, F. Zhang, X. Liu, Z. Su, J. Hu and Y. Qian, Nat. Nanotechnol., 2021, 16, 1371–1377 CrossRef CAS PubMed.
- J. Yoon, W. Choi, T. Kim, H. Kim, Y. S. Choi, J. M. Kim and W.-S. Yoon, J. Energy Chem., 2021, 53, 276–284 CrossRef.
- J. Qin, H. Liu, P. Zou, R. Zhang, C. Wang and H. L. Xin, J. Am. Chem. Soc., 2022, 144, 2197–2207 CrossRef CAS PubMed.
- Y. Ren, Y. Tang, L. Zhang, X. Liu, L. Li, S. Miao, D. Sheng Su, A. Wang, J. Li and T. Zhang, Nat. Commun., 2019, 10, 4500 CrossRef PubMed.
- W. Yang, Y. Zhu, F. You, L. Yan, Y. Ma, C. Lu, P. Gao, Q. Hao and W. Li, Appl. Catal., B, 2018, 233, 184–193 CrossRef CAS.
- F. Li, Y.-L. Liu, G.-G. Wang, D. Yan, G.-Z. Li, H.-X. Zhao, H.-Y. Zhang and H.-Y. Yang, J. Mater. Chem. A, 2021, 9, 9675–9684 RSC.
- T. Gao, M. Glerup, F. Krumeich, R. Nesper, H. Fjellvåg and P. Norby, J. Phys. Chem. C, 2008, 112, 13134–13140 CrossRef CAS.
- L. Yan, C. Zhu, J. Hao, X. Liang, Y. Bai, Q. Hu, B. Tan, B. Liu, X. Zou and B. Xiang, Adv. Funct. Mater., 2021, 31, 2102693 CrossRef CAS.
- X. Zhang, C. Chen, X. Chen, L. Wang, T. Huang and A. Yu, ChemistrySelect, 2019, 4, 7455–7462 CrossRef CAS.
- P. K. Gupta, A. Bhandari, S. Saha, J. Bhattacharya and R. G. S. Pala, J. Phys. Chem. C, 2019, 123, 22345–22357 CrossRef CAS.
- S. Liang, F. Teng, G. Bulgan, R. Zong and Y. Zhu, J. Phys. Chem. C, 2008, 112, 5307–5315 CrossRef CAS.
- X. Shao, J. Ma, G. Wen and J. Yang, Chin. Sci. Bull., 2010, 55, 802–808 CrossRef CAS.
- S. B. Scott, J. E. Sørensen, R. R. Rao, C. Moon, J. Kibsgaard, Y. Shao-Horn and I. Chorkendorff, Energy Environ. Sci., 2022, 15, 1988–2001 RSC.
- Y. Zhang, X. Zhu, G. Zhang, P. Shi and A.-L. Wang, J. Mater. Chem. A, 2021, 9, 5890–5914 RSC.
- L. K. McLeod, G. H. Spikes, R. J. Kashtiban, M. Walker, A. V. Chadwick, J. D. Sharman and R. I. Walton, Dalton Trans., 2020, 49, 2661–2670 RSC.
- L. C. Seitz, C. F. Dickens, K. Nishio, Y. Hikita, J. Montoya, A. Doyle, C. Kirk, A. Vojvodic, H. Y. Hwang and J. K. Norskov, Science, 2016, 353, 1011–1014 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, additional materials characterization data, electrochemical results, and computation details. See https://doi.org/10.1039/d2ta05335g |
| ‡ Equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2022 |