Regulation of the integrated hydrogen storage properties of magnesium hydride using 3D self-assembled amorphous carbon-embedded porous niobium pentoxide†
Abstract
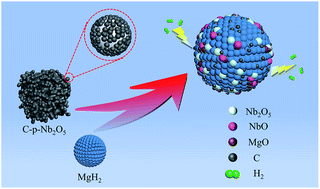
Three different types of porous niobium pentoxide, namely, porous niobium pentoxide (p-Nb2O5), carbon-embedded porous niobium pentoxide (C-p-Nb2O5), and non-porous niobium pentoxide (np-Nb2O5), were prepared by the wet chemical method and employed to improve the hydrogen storage properties of magnesium hydride (MgH2). It was found that C-p-Nb2O5 played an important role in improving the hydrogen storage performance of MgH2. The C-p-Nb2O5-doped and p-Nb2O5-doped MgH2 samples had the same dehydrogenation temperature of 181 °C, which was lower than those of the np-Nb2O5-doped (200 °C) and undoped MgH2 (300 °C) samples. The catalytic effect of C-p-Nb2O5 on the hydrogen storage performance of MgH2 was better than those of p-Nb2O5 and np-Nb2O5. The dehydrogenation apparent activation energy of C-p-Nb2O5-doped MgH2 (69.3 kJ mol−1) was 7.4 kJ mol−1, 12.8 kJ mol−1, and 72.2 kJ mol−1 lower than those of p-Nb2O5-doped MgH2, np-Nb2O5-doped MgH2, and undoped MgH2, respectively, and their catalytic effect on the hydrogen storage performance of MgH2 was in the order of C-p-Nb2O5 > p-Nb2O5 > np-Nb2O5. The mechanism analysis indicated that partial Nb2O5 reacted with MgH2 to form NbO and MgO during the milling process. Under the combined effect of C, Nb2O5, and in situ formed NbO, the C-p-Nb2O5-doped MgH2 sample manifested an excellent comprehensive hydrogen storage performance.


 Please wait while we load your content...
Please wait while we load your content...