Open Access Article
Open Access ArticleHighly stable Pebax® Renew® thin-film nanocomposite membranes with metal organic framework ZIF-94 and ionic liquid [Bmim][BF4] for CO2 capture†
Lidia
Martínez-Izquierdo
 ab,
Carlos
Téllez
ab,
Carlos
Téllez
 ab and
Joaquín
Coronas
ab and
Joaquín
Coronas
 *ab
*ab
aInstituto de Nanociencia y Materiales de Aragón (INMA), CSIC-Universidad de Zaragoza, Zaragoza 50018, Spain. E-mail: coronas@unizar.es
bChemical and Environmental Engineering Department, Universidad de Zaragoza, Zaragoza 50018, Spain
First published on 30th August 2022
Abstract
Ionic liquid (IL) [Bmim][BF4] and metal organic framework (MOF) ZIF-8 and ZIF-94 nanoparticles were incorporated into polymer Pebax® Renew® 30R51 to obtain highly efficient thin-film nanocomposite (TFN) membranes, ca. 300 nm thick. ZIF-94 particles were synthesized via a solvent assisted ligand exchange (SALE) from nano ZIF-8. To fabricate the membranes, the weight percentage of [Bmim][BF4] was firstly varied from 5 to 20 wt% to find the optimal IL content (10 wt%). Besides, ZIF-8 and ZIF-94 nanoparticles were separately incorporated (from 10 to 20 wt%) into the Pebax®/IL matrix to enhance the CO2 separation performance. Compared with the pristine Pebax® thin film composite (TFC) membrane, the IL (10 wt%) increased the CO2 permeance by 27% to 629 GPU and the CO2/N2 separation selectivity by 7% to 29 due to the enhancement in CO2 mass transport. After the incorporation of ZIFs, the CO2 permeance increased by 51% to 751 GPU and by 65% to 819 GPU, with 15 wt% loadings of ZIF-8 and ZIF-94, respectively, although the CO2/N2 separation selectivity decreased by 7% to 25 in both cases. Additional characterization was also carried out by the calculation of the permeation apparent activation energies for CO2 and N2 as well as the study of the long-term stability for up to 18 days. This demonstrated that the incorporation of the MOF enhanced the stability of the IL in the membrane, rendering it 55% and 35% more permeable (611 GPU) than the bare polymer and the membrane with only IL, respectively, while maintaining a CO2/N2 separation selectivity of 25.
1. Introduction
To reach the international target of net-zero CO2 emissions by 2050, the use of green and renewable energy has drawn remarkable attention. Moreover, the development of technologies capable of separating and capturing CO2 is also the scope of many studies.1–3 Among the existing methods of CO2 separation (e.g. amine absorption, cryogenic distillation and adsorption), membrane technology has many advantages such as simple processing equipment, operating flexibility, high reliability, small footprint, low energy requirement and environment friendliness.4–7 However, current dense membranes suffer from very low CO2 permeance, which makes them noncompetitive for industrial applications.8 To achieve efficient separations, exceeding the Robeson trade-off relationship between permeability and selectivity,9 thin film composite membranes (TFC) must be prepared with a very thin selective layer (with a thickness lower than 1 μm).In addition, the introduction of nanoparticles with molecular sieving properties within the polymer matrix is also regarded to improve the gas separation performance.10,11 A variety of nano-fillers have been proved to enhance the separation performance of membranes, e.g. carbon derivatives,12–14 zeolites15,16 and metal–organic frameworks (MOFs).17–20 Among them, MOFs and in particular zeolitic imidazolate frameworks (ZIFs), a sub-class of MOF, are being widely studied due to their improved affinity to the polymer matrix.21 Such increased compatibility in MOF-type fillers is possible due to the presence of organic linkers within their structure.22 Besides solid nano-fillers, the incorporation of liquids (e.g. ionic liquids, ILs) with increased mass transport in comparison to the polymer matrix is also the subject of many studies.23,24 Such increment in the mass transport velocity allows higher permeances of CO2 through the membrane and more efficient separations.25 Interestingly, this approach has been followed to prepare a mix of both solid particles and ILs, which is now gaining special attention due to the possibility of boosting the gas separation performance of membranes by combining their properties. Lu et al.26 prepared PIM-1 mixed matrix membranes (MMMs) with an IL-modified UiO-66-NH2 filler. They found that the IL improved not only the hydrophobicity of UiO-66-NH2, thus facilitating the dispersion of the particles into the polymer, but also the affinity between the MOF and polymer. By using this strategy, they were able to reduce the non-selective interfacial defects with the corresponding enhancement of the gas separation behavior. Attempts to combine the advantages of solid and liquid additives have also been made with carbonaceous based particles. Huang et al.27 prepared a Pebax®/ionic liquid modified graphene oxide (GO-IL) MMM with enhanced CO2 separation performance. They found that the IL improved both the CO2 solubility and the CO2/gas selectivity of the MMMs. This allowed them to increase the CO2/N2 selectivity and the CO2 permeability over 90% and 50%, respectively, compared to the pristine membrane. Jomekian et al.28 prepared surface IL-modified Pebax® 1657 membranes filled with ZIF-8 particles. These MMMs with a 13 μm thick Pebax® layer on a PES support had an insignificant content of IL, whose mission was to functionalize the Pebax® surface. This caused an increase in the CO2 selectivity in the membranes with ZIF-8 due to the better interaction between the components of the MMM. Such improvement in the interaction of the filler with the polymer due to the presence of an ionic liquid has also been reported in other works.29,30
ZIF-94, also named as SIM-1, is a zeolitic imidazolate framework synthesized from Zn atoms and 4-methyl-5-imidazolate-carboxaldehyde linkers sharing the same SOD type topology as ZIF-8.31,32 In the present work, ZIF-94 nanoparticles were combined with the anionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4] and incorporated into a Pebax® Renew® polymer to prepare thin film nanocomposite (TFN) membranes. These, unlike a typical dense MMM, are supported MMMs whose thin selective layer incorporates nanoparticles. Pebax® is a commercial brand of block copolymer elastomers built up of rigid polyamide blocks and soft polyether blocks (PEBA). Pebax® Renew® code was chosen for this project due to its renewable nature (it contains 41% of renewable carbon, measured by ASTM D6866 as reported by Arkema in the corresponding data sheet), and similar composition to highly studied Pebax® 1657, thus anticipating similar separation properties. As previously noted by Neves et al.33 and Jiang et al.,34 the IL [Bmim][BF4] was selected over other imidazolium-based ionic liquids due to its improved performance in gas separation, ideal to demonstrate the membrane concept developed in this work. To the best of our knowledge this is a non-studied system;35,36 the closest situation includes Pebax® 1657/graphene oxide-IL TFN membranes, whose operation stability was studied for only up to 28 h.27,37 Besides, it is worth mentioning that the ZIF-94 crystals used for this research were prepared via a solvent assisted ligand exchange (SALE) reaction using 1-butanol as reaction medium and nano-ZIF-8 as precursor material. With this strategy, the obtained ZIF has the controlled particle size of ZIF-8 and the high CO2-philicity of ZIF-94 due to the aldehyde group in its ligand. This SALE method avoids the use of other toxic solvents such as tetrahydrofuran (THF) or dimethylformamide (DMF),38 while taking advantage of the easy control of ZIF-8 synthesis in terms of particle size and geometry. ILs and MOFs have been previously incorporated together into PEBA membranes for gas separation.23,39,40 However, the vast majority of reports available concerning this topic are related to self-supported MMMs. The intent of this research is to prepare a new generation of TFN membranes for CO2/N2 and CO2/CH4 separation with improved gas separation performance over time.
2. Experimental
2.1. Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) and 2-methylimidazole (2-mIm) were purchased from sigma Aldrich. 4-Methyl-5-imidazole-carboxyaldehyde was purchased from Acros Chemicals. The ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4] (Scheme 1), was purchased from Fisher Scientific, Spain. Polysulfone (Udel® P-3500 LCD) was purchased from Solvay Advanced Polymers. Poly[1-(trimethylsilyl)prop-1-yne] (PTMSP) was purchased from Fluorochem, United Kingdom. Polyether-block-amide, Pebax® Renew® 30R51 (with a percentage of renewable carbon of 41%) in the form of pellets was kindly provided by Arkema, France. This is a thermoplastic elastomer made from flexible polyether and rigid polyamide based on renewable resources. The solvents, methanol (MeOH), N-methyl-2-pyrrolidone (NMP), 1-propanol (1-PrOH) and 1-butanol (1-ButOH) were purchased from Análisis Vínicos, Panreac, Labbox and Scharlab, Spain, respectively. All gases used for the separation tests were of research grade (greater than 99.995% of purity) and supplied by Abelló Linde S. A., Spain. All gases, polymers and solvents were used as received.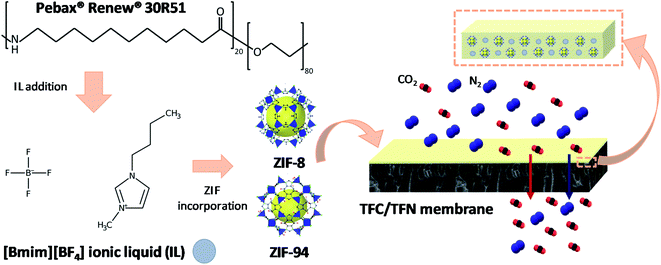 | ||
| Scheme 1 Schematic diagram of Pebax 30R51, [Bmim][BF4] IL and ZIF-8 and ZIF-94 structures (corresponding to hybrid ZIF ZIF-94/ZIF-8) and the gas separation mechanism through a TFC/TFN membrane. | ||
2.2. Methods
The solvent assisted ligand exchange (SALE) reaction was carried out according to a previously reported method by Marti et al.43 Briefly, 0.323 g of 4-methyl-5-carboxyaldehyde (2.94 mmol) were first dissolved in 20 mL of 1-ButOH. Then, 100 mg of nano ZIF-8 were suspended in the precursor solution and stirred at RT for 24 h. The resulting product was collected by centrifugation at 9000 rpm for 10 min and washed several times with fresh 1-ButOH under the same conditions. The final crystals were dried and activated at 40 °C overnight. The ZIF-94 particles obtained this way were named ZIF-94 (SALE).
A PTMSP gutter layer was spin-coated (Fig. 1) onto the PSF supports to avoid the penetration of the selective layer. Firstly, a PTMSP solution was prepared dissolving at room temperature the polymer in n-hexane in a concentration of 2 wt%. Once dissolved, 0.6 mL of this solution was poured onto the support, previously attached to the spinner by vacuum, and spun at 2500 rpm for 20 s. The supports with the gutter layer were placed in an oven at 40 °C for 1 h to evaporate the residual solvent.
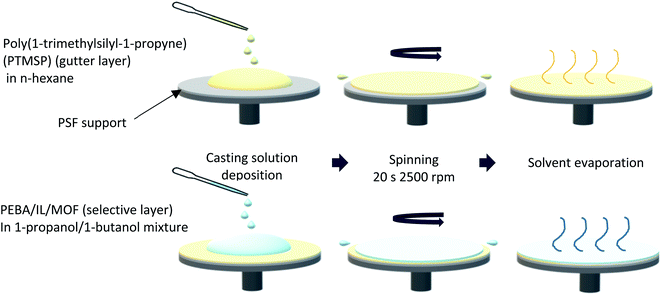 | ||
| Fig. 1 Spin-coating method: from the coating of the PTMSP gutter layer to that of the PEBA/IL/ZIF in one step. | ||
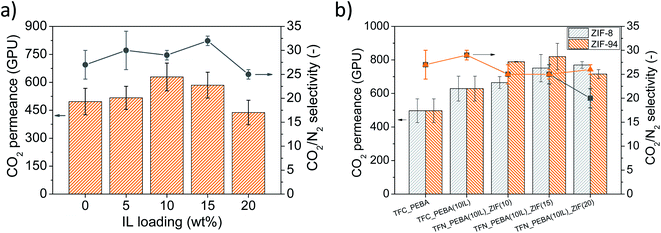
The final selective layer was also spin-coated onto the PTMSP/PSF supports at 2500 rpm for 20 s. For this purpose, a Pebax® Renew® solution was prepared dissolving under reflux 0.2 g of the polymer in 9.8 g of a 1-PrOH/1-ButOH (3/1 v/v) mixture at 80 °C for 2 h. To avoid gelation, the polymer solution was immersed in a water bath at 45 °C. Finally, 0.6 mL of this solution was poured onto the PTMSP/PSF support and spun to obtain the Pebax®Renew®/PTMSP/PSF TFC membrane. Similarly, Pebax®Renew® (IL)/PTMSP/PSF membranes were prepared by adding the IL (from 5 to 20 wt%, respect to the polymer) to the Pebax® solution once cooled down. Solutions of Pebax® with the IL were stirred at 45 °C for 1 h before spinning. The TFC membranes prepared this way were abbreviated as TFC_PEBA(XIL), where X is the IL concentration. After testing the TFC_PEBA(XIL) membranes in the gas separation set up, the optimal condition (10 wt% of IL, see section 3.3.1.) was selected to prepare the TFN membranes with the nanocrystals of ZIF-8 and ZIF-94 prepared by the SALE reaction. In this case, the polymer was dissolved in 2/3 of the total solvent under the same conditions while different amounts of ZIF-8 and ZIF-94 particles (from 10 to 20 wt%, respect to the polymer) were suspended in the remaining solvent (1/3 of the total) using an ultrasonic bath. Once the polymer was dissolved and cooled down to 40–45 °C, the IL was added to the solution and stirred for 5 min before incorporating the ZIF suspension. Casting suspensions with the IL and ZIFs were stirred for 1 h at 45 °C before spinning. The membranes prepared this way were named as TFN_PEBA(10IL)_ZIF8(Y) and TFN_PEBA(10IL)_ZIF94(Y), where Y is the wt% concentration of ZIF-8 and ZIF-94. All the membranes were placed in an oven at 40 °C for 18 h after spinning to remove any residual solvent.
3. Results
3.1. Characterization of ZIF-8 and ZIF-94
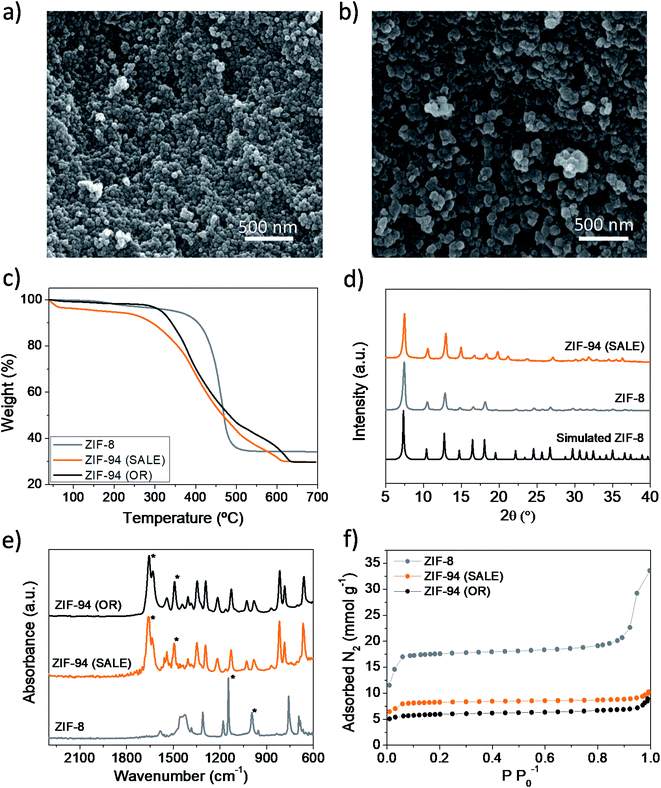
ZIF-8 and ZIF-94 were synthesized in order to be used as loadings for Pebax® Renew® thin film nanocomposite (TFN) membranes. As seen in Fig. 2a and b, the average particle sizes of synthesized ZIF-8 and ZIF-94 (SALE) were 30 ± 5 nm and 48 ± 6 nm, respectively. Furthermore, the ZIF-94 particles have a less round shape and a larger particle size which may be related to an Ostwald ripening effect during the SALE.45 To confirm the ligand exchange, TGA analyses were carried out from 35 to 700 °C under air atmosphere. Results (Fig. 2c) revealed that the particles synthesized from ZIF-8 (ZIF-94 (SALE)) had the same degradation behavior that the ones synthesized by the original route (ZIF-94 (OR)), which besides differs from the degradation behavior of ZIF-8. While the maximum degradation of ZIF-94 nanoparticles takes place between 375 °C and 400 °C, the one of ZIF-8 happens at a higher temperature, ca. 450 °C. It is also worth mentioning that the ZIF-8 weight loss is more abrupt than those of ZIF-94 OR and SALE. Furthermore, the weight loss between 100–200 °C in the ZIF-94 (OR) thermogram suggests that there is still some remaining solvent from the ZIF synthesis. Conversely, the ZIF-94 (SALE) particles do not present such weight loss, indicating that the powder was successfully activated, probably due to the washing produced during the SALE process. The crystallinity and purity of the ZIF particles were confirmed by XRD. The patterns of the simulated ZIFs and the synthesized ones are plotted together for comparison in Fig. 2d. As seen in this figure, the peak positions match well with those of the simulated ZIF-8. In fact, as both ZIFs share the same SOD type structure, the simulated patterns of ZIF-8 and ZIF-94 can be considered the same.32,46 The FTIR-ATR spectra of ZIFs are depicted in Fig. 2e, revealing a similar spectrum for ZIF-94 (SALE) than for ZIF-94 (OR), with the main bands of ZIF-94 at 1660 cm−1, which corresponds to the aldehyde group (–CHO),47 and 1496 cm−1, related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Besides the similar FTIR spectra of ZIF-94 (SALE) and (OR), the absence of the main bands of ZIF-8 at 1147 cm−1 and 993 cm−1 (both corresponding to the C–N bond)48 agree with the successful ligand exchange. The N2 adsorption isotherms and BET SSA values also confirm the ligand exchange (Fig. 2f). In this case, the BET SSA decreases from 1350 cm2 g−1 for the ZIF-8 to 464 cm2 g−1 for the ZIF-94 (OR) and to 645 cm2 g−1 for the ZIF-94 (SALE), which is in accordance with the literature for synthesized ZIF-94 particles.47,49 However, these values suggest that the conversion of ZIF-8 into ZIF-94 was high but not complete with a remain of the ZIF-8 composition responsible for the larger value of BET SSA for ZIF-94 (SALE) as compared to that of the as-made ZIF-94. Furthermore, Fig. 2f reveals the type I IUPAC classification of adsorption isotherms for both products, thus indicating dominant microporosity of these materials.50
C bond. Besides the similar FTIR spectra of ZIF-94 (SALE) and (OR), the absence of the main bands of ZIF-8 at 1147 cm−1 and 993 cm−1 (both corresponding to the C–N bond)48 agree with the successful ligand exchange. The N2 adsorption isotherms and BET SSA values also confirm the ligand exchange (Fig. 2f). In this case, the BET SSA decreases from 1350 cm2 g−1 for the ZIF-8 to 464 cm2 g−1 for the ZIF-94 (OR) and to 645 cm2 g−1 for the ZIF-94 (SALE), which is in accordance with the literature for synthesized ZIF-94 particles.47,49 However, these values suggest that the conversion of ZIF-8 into ZIF-94 was high but not complete with a remain of the ZIF-8 composition responsible for the larger value of BET SSA for ZIF-94 (SALE) as compared to that of the as-made ZIF-94. Furthermore, Fig. 2f reveals the type I IUPAC classification of adsorption isotherms for both products, thus indicating dominant microporosity of these materials.50
3.2. Characterization of membranes
The thicknesses of the PTMSP and Pebax® layers were measured by SEM. Cross-sections of the membranes are depicted in Fig. 3a–d. As observed in all images, a very thin layer of Pebax® Renew® (300 nm) was coated on top of a 1 μm thick PTMSP gutter layer. The PTMSP gutter layer is placed between the support and the selective layer to avoid the polymer penetration of the latest into the support,51 which would be detrimental for the final gas separation performance unnecessarily increasing the transport resistance. Indeed, PTMSP constitutes a poorly selective and highly permeable glassy polymeric material and it is expected that its contribution to the final resistance to the gas permeation is negligible.52 Further characterization was carried out to study the stability and crystallinity of the membranes by TGA, DTG and XRD analyses. With this purpose, a dense Pebax® Renew® membrane was prepared via casting-solution and the results are depicted in Fig. S1a and b,† respectively. As seen in Fig. S1a,† the Pebax® Renew® polymer is stable up to 300 °C and it is degraded completely at 540 °C. In this figure, two degradation steps are appreciable. The first one (from 300 °C to 460 °C) is related to the major thermal degradation of the polymer, whereas the second (from 460 °C to 540 °C) corresponds to the carbonization of the degraded polymer chains.53 The XRD pattern shown in Fig. S1b† depicts the semicrystalline nature of Pebax® type copolymers.17,54,55 In the case of this renewable code, three main crystalline peaks are noticeable in the diffractogram at 7.6°, 20.4° and 24.3° 2θ values, the first and second corresponding to the PEO and the third to the PA11 segments.56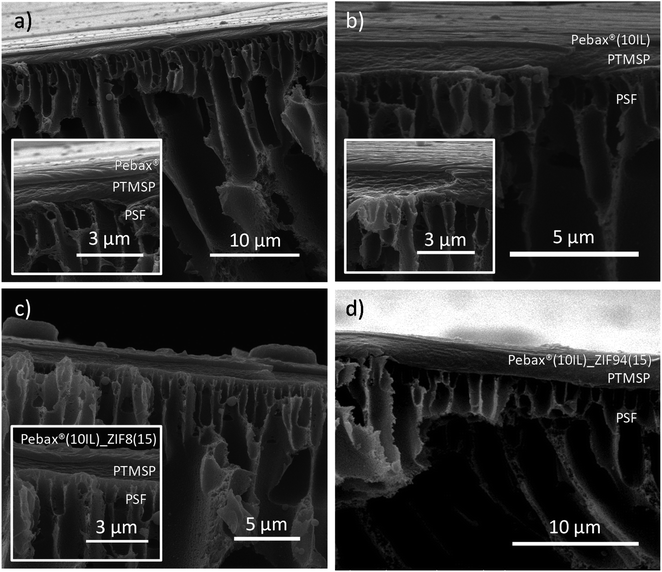 | ||
| Fig. 3 Cross-section SEM images of some of the Pebax® Renew® TFC membranes prepared in this work. (a) TFC_PEBA, (b) TFC_PEBA(10IL), (c) TFN_PEBA(10IL)_ZIF8(15) and (d) TFN_PEBA(10IL)_ZIF94(15). | ||
3.3. Gas separation performance
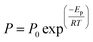 | (1) |
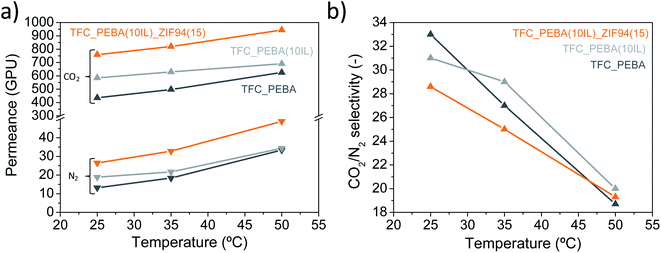 | ||
| Fig. 5 Gas separation performance as a function of temperature. Measured at 25, 35 and 50 °C and 3 bar. (a) CO2 and N2 permeances and (b) CO2/N2 selectivity. | ||
| Membrane | E p CO2 | E p N2 | Ref |
|---|---|---|---|
| kJ mol−1 | |||
| Pebax® 1657 | 13.3 | 30.4 | 66 |
| Pebax® 1657 | 14.9 | 28.0 | 64 |
| Pebax® 1657 | 14.6 | 33.6 | 67 |
| Pebax® 2533 | 16.7 | 27.2 | 68 |
| Pebax® 2533 | 18.2 | 31.0 | 69 |
| Pebax® 3533 | 14.2 | 29.6 | 70 |
| Pebax® 1074 | 13.4 | 30.3 | 44 |
| TFC_PEBA | 11.7 | 30.1 | This work |
| TFC_PEBA(10IL) | 5.3 | 19.6 | This work |
| TFC_PEBA(10IL)_ZIF94(15) | 7.0 | 19.7 | This work |
| Membrane | CO2 permeance (GPU) | CO2/N2 selectivity | Ref |
|---|---|---|---|
| a Hollow fibers. | |||
| Pebax®2533/[Bmim][BF4] | 101 | 42 | 78 |
| Pebax®1657/GO-IL | 905 | 45 | 27 |
| Pebax®1657/[Emim][BF4]/GOa | 642 | 34 | 37 |
| Pebax®1657/[Emim][BF4]a | 300 | 36 | 79 |
| Pebax®2533/TSIL | 250 | 30 | 80 |
| TFC_PEBA | 497 | 27 | This work |
| TFC_PEBA(10IL) | 629 | 29 | This work |
| TFC_PEBA(10IL)_ZIF8(15) | 751 | 25 | This work |
| TFC_PEBA(10IL)_ZIF94(15) | 819 | 25 | This work |
4. Conclusions
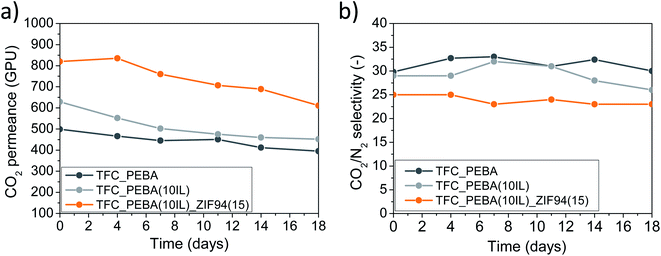
Pebax® Renew® 30R51 TFC membranes and ZIF-8 or ZIF-94/IL/Pebax® Renew® TFN membranes were prepared and characterized for CO2/N2 separation. ZIF-94 was prepared via a solvent assisted ligand exchange (SALE) reaction using ZIF-8 as a precursor material suspended in 1-butanol. The high extension of the ligand exchange was confirmed by TGA, DTG, XRD, FTIR and BET analyses. The addition of [Bmim][BF4] into the Pebax® Renew® composite membranes enhanced the CO2 separation performance of the membranes up to 10 wt% of loading, reaching a CO2 permeance of 629 GPU and a CO2/N2 selectivity of 29, which means increases of 27% and 11% compared with the CO2 permeance and the CO2/N2 separation selectivity of the pristine membrane, respectively. The incorporation of ZIF-8 or ZIF-94 particles into the IL/Pebax® matrix also improved the CO2 permeance of the membranes over the ones which only had IL, which was attributed to the improved compatibility between ZIFs and the polymer matrix due to the presence of the IL. For both ZIFs, the best gas separation results were obtained at 15 wt% of ZIF loading and 10 wt% of IL, reaching CO2 permeances of 751 GPU and 819 GPU for ZIF-8 and ZIF-94, respectively, together with a CO2/N2 selectivity of 25 in both cases. It is worth mentioning that it was for up to 15 wt% of ZIF loading that the membranes with ZIF-94 were more permeable than those with ZIF-8. This behavior was attributed to the CO2-philic nature of ZIF-94, due to its aldehyde group which in turn increases its hydrophilicity and compatibility with the polymer and the IL. In addition, the apparent activation energies of permeation were also calculated for the optimal membranes, obtaining similar results to those reported in the literature for Pebax® type copolymer with the pristine TFC membrane, but lower values with the membranes with IL and IL/ZIF-94. These results were related to the increment of CO2 and N2 permeation, clearly supporting the positive influence of IL and ZIF on the diffusion and solubility properties of the membranes with respect to the pure polymer.The long-term stability of the membranes was studied for 18 days. Results indicated that the IL affected the stability of the membrane increasing the CO2 permeance loss from 20% to 28%. However, the incorporation of ZIF-94 partially mitigated such deterioration reaching a CO2 permeance loss of 25% after 18 days of testing. In spite of the fact that the long-term stability of the membranes was affected by the use of PTMSP as a gutter layer, the CO2 permeance loss was far from previous results found in the literature. In any event, after the 18 days of continuous performance, the membrane with IL and ZIF-94 was 55% and 35% more CO2 permeable than the bare polymer and the membrane with only IL, respectively, maintaining a CO2/N2 separation selectivity of 25.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Grants PID2019-104009RB-I00 funded by MCIN/AEI/10.13039/501100011033 is gratefully acknowledged (Agencia Estatal de Investigación (AEI) and MCIN (Ministerio de Ciencia e Innovación), Spain). Grant T43-20R financed by the Aragón Government is gratefully acknowledged. L. Martínez-Izquierdo also thanks the Aragón Government (DGA) for her PhD grant. The authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación (SAI) and the use of instrumentation as well as the technical advice provided by the National Facility ELECMI ICTS, node “Laboratorio de Microscopias Avanzadas” at the University of Zaragoza.References
- Y. Zhang, J. Sunarso, S. Liu and R. Wang, Int. J. Greenhouse Gas Control, 2013, 12, 84–107 CrossRef CAS.
- N. Saini and K. Awasthi, Sep. Purif. Technol., 2022, 282, 120029 CrossRef CAS.
- N. H. Solangi, A. Anjum, F. A. Tanjung, S. A. Mazari and N. M. Mubarak, J. Environ. Chem. Eng., 2021, 9, 105860 CrossRef CAS.
- B. Zhu, S. He, Y. Wu, S. Li and L. Shao, Engineering, DOI: DOI:10.1016/J.ENG.2022.03.016.
- S. He, B. Zhu, X. Jiang, G. Han, S. Li, C. H. Lau, Y. Wu, Y. Zhang and L. Shao, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2114964119 CrossRef CAS PubMed.
- S. Ding, X. Li, S. Ding, W. Zhang, R. Guo and J. Zhang, Sep. Purif. Technol., 2020, 239, 116539 CrossRef CAS.
- A. Brunetti, F. Scura, G. Barbieri and E. Drioli, J. Membr. Sci., 2010, 359, 115–125 CrossRef CAS.
- W. Yave, A. Car, J. Wind and K.-V. Peinemann, Nanotechnology, 2010, 21, 7 CrossRef PubMed.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- A. Guo, Y. Ban, K. Yang, Y. Zhou, N. Cao, M. Zhao and W. Yang, J. Membr. Sci., 2020, 601, 117880 CrossRef.
- J. Dechnik, J. Gascon, C. J. Doonan, C. Janiak and C. J. Sumby, Angew. Chem., Int. Ed., 2017, 56, 9292–9310 CrossRef CAS PubMed.
- F. Amirkhani, M. Mosadegh, M. Asgharia and M. J. Parnian, Polym. Test., 2020, 82, 106285 CrossRef CAS.
- D. Peng, S. Wang, Z. Tian, X. Wu, Y. Wu, H. Wu, Q. Xin, J. Chen, X. Cao and Z. Jiang, J. Membr. Sci., 2017, 522, 351–362 CrossRef CAS.
- T.-C. Huang, Y.-C. Liu, G.-S. Lin, C.-H. Lin, W.-R. Liu and K.-L. Tung, J. Membr. Sci., 2020, 602, 117946 CrossRef CAS.
- S. Zhang, Y. Zheng, Y. Wu and B. Zhang, J. Appl. Polym. Sci., 2021, 138, 51336 CrossRef CAS.
- Y. Zheng, Y. Wu, B. Zhang and Z. Wang, J. Appl. Polym. Sci., 2020, 48398 CrossRef CAS.
- J. Sánchez-Laínez, I. Gracia-Guillé, B. Zornoza, C. Téllez and J. Coronas, New J. Chem., 2019, 43, 312–319 RSC.
- P.-H. Tang, P. B. So, W.-H. Li, Z.-Y. Hui, C.-C. Hu and C.-H. Lin, Membranes, 2021, 11, 404 CrossRef CAS PubMed.
- Y. Shi, S. Wu, Z. Wang, X. Bi, M. Huang, Y. Zhang and J. Jin, Sep. Purif. Technol., 2021, 277, 119449 CrossRef CAS.
- M. Benzaqui, M. Wahiduzzaman, H. Zhao, M. R. Hasan, T. Steenhaut, A. Saad, J. Marrot, E. Normand, J.-M. Grenèche, N. Heymans, G. De Weireld, A. Tissot, W. Shepard, Y. Filinchuk, S. Hermans, F. Carn, M. Manlankowska, C. Téllez, J. Coronas, G. Maurin, N. Steunou and C. Serre, J. Mater. Chem. A, 2022, 10, 8535–8545 RSC.
- M. Benzaqui, R. Semino, N. Menguy, F. Carn, T. Kundu, J.-M. Guigner, N. B. Mckeown, K. J. Msayib, M. Carta, R. Malpass-Evans, ⊥ Clément, C. Le Guillouzer, G. Clet, N. A. Ramsahye, C. Serre, G. Maurin and N. Steunou, ACS Appl. Mater. Interfaces, 2016, 8, 27311–27321 CrossRef CAS PubMed.
- M. van Essen, R. Thür, L. van den Akker, M. Houben, I. F. J. Vankelecom, K. Nijmeijer and Z. Borneman, J. Membr. Sci., 2021, 637, 119642 CrossRef CAS.
- Z. Guo, W. Zheng, X. Yan, Y. Dai, X. Ruan, X. Yang, X. Li, N. Zhang and G. He, J. Membr. Sci., 2020, 605, 118101 CrossRef CAS.
- F. Galiano, R. Mancuso, L. Guazzelli, M. Mauri, C. Chiappe, R. Simonutti, A. Brunetti, C. S. Pomelli, G. Barbieri, B. Gabriele and A. Figoli, J. Membr. Sci., 2021, 635, 119479 CrossRef CAS.
- C. Hermida-Merino, F. Pardo, G. Zarca, J. M. M. Araújo, A. Urtiaga, M. M. Piñeiro and A. B. Pereiro, Nanomater, 2021, 11, 607 CrossRef CAS PubMed.
- J. Lu, X. Zhang, L. Xu, G. Zhang, J. Zheng, Z. Tong, C. Shen and Q. Meng, Membr, 2021, 11, 35 CrossRef CAS PubMed.
- G. Huang, A. P. Isfahani, A. Muchtar, K. Sakurai, B. B. Shrestha, D. Qin, D. Yamaguchi, E. Sivaniah and B. Ghalei, J. Membr. Sci., 2018, 565, 370–379 CrossRef CAS.
- A. Jomekian, B. Bazooyar, R. M. Behbahani, T. Mohammadi and A. Kargari, J. Membr. Sci., 2017, 524, 652–662 CrossRef CAS.
- C. Casado-Coterillo, A. Fernández-Barquín, B. Zornoza, C. Téllez, J. Coronas and Á. Irabien, RSC Adv., 2015, 5, 102350–102361 RSC.
- M. T. Vu, R. Lin, H. Diao, Z. Zhu, S. K. Bhatia and S. Smart, J. Membr. Sci., 2019, 587, 117157 CrossRef CAS.
- S. Aguado, J. Canivet and D. Farrusseng, Chem. Commun., 2010, 46, 7999–8001 RSC.
- W. Morris, N. He, K. G. Ray, P. Klonowski, H. Furukawa, I. N. Daniels, Y. A. Houndonougbo, M. Asta, O. M. Yaghi and B. B. Laird, J. Phys. Chem. C, 2012, 116, 24084–24090 CrossRef CAS.
- L. A. Neves, N. Nemestóthy, V. D. Alves, P. Cserjési, K. Bélafi-Bakó and I. M. Coelhoso, Desalination, 2009, 240, 311–315 CrossRef CAS.
- Y. Jiang, Y. Wu, W. Wang, L. Li, Z. Zhou and Z. Zhang, Chin. J. Chem. Eng., 2009, 17, 594–601 CrossRef CAS.
- X. Yan, S. Anguille, M. Bendahan and P. Moulin, Sep. Purif. Technol., 2019, 222, 230–253 CrossRef CAS.
- K. Friess, P. Izák, M. Kárászová, M. Pasichnyk, M. Lanč, D. Nikolaeva, P. Luis and J. C. Jansen, Membr, 2021, 11, 97 CrossRef CAS PubMed.
- W. Fam, J. Mansouri, H. Li, J. Hou and V. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 7389–7400 CrossRef CAS PubMed.
- T. Johnson, M. M. Łozińska, A. F. Orsi, P. A. Wright, S. Hindocha and S. Poulston, Green Chem., 2019, 21, 5665–5670 RSC.
- H. Li, L. Tuo, K. Yang, H. K. Jeong, Y. Dai, G. He and W. Zhao, J. Membr. Sci., 2016, 511, 130–142 CrossRef CAS.
- Z. Yang, Y. Ying, Y. Pu, D. Wang, H. Yang and D. Zhao, Ind. Eng. Chem. Res., 2022, 61, 7626–7633 CrossRef CAS.
- J. Sánchez-Laínez, B. Zornoza, S. Friebe, J. Caro, S. Cao, A. Sabetghadam, B. Seoane, J. Gascon, F. Kapteijn, C. Le Guillouzer, G. Clet, M. Daturi, C. Téllez and J. Coronas, J. Membr. Sci., 2016, 515, 45–53 CrossRef.
- D. Madhav, M. Malankowska and J. Coronas, New J. Chem., 2020, 44, 20449–20457 RSC.
- A. M. Marti, M. Van and K. J. Balkus, J. Porous Mater., 2014, 21, 889–902 CrossRef CAS.
- L. Martínez-Izquierdo, M. Malankowska, C. Téllez and J. Coronas, J. Environ. Chem. Eng., 2021, 9, 105624 CrossRef.
- C. W. Tsai, J. W. Niemantsverdriet and E. H. G. Langner, Microporous Mesoporous Mater., 2018, 262, 98–105 CrossRef CAS.
- M. Etxeberria-Benavides, O. David, T. Johnson, M. M. Łozińska, A. Orsi, P. A. Wright, S. Mastel, R. Hillenbrand, F. Kapteijn and J. Gascon, J. Membr. Sci., 2018, 550, 198–207 CrossRef CAS.
- F. Cacho-Bailo, M. Etxeberría-Benavides, O. Karvan, C. Téllez and J. Coronas, CrystEngComm, 2017, 19, 1545–1554 RSC.
- V. Berned-Samatán, C. Rubio, A. Galán-González, E. Muñoz, A. M. Benito, W. K. Maser, J. Coronas and C. Téllez, J. Membr. Sci., 2022, 652, 120490 CrossRef.
- M. R. Hasan, L. Paseta, M. Malankowska, C. Téllez and J. Coronas, Adv. Sustainable Syst., 2021, 2100317 Search PubMed.
- F. Akbari Beni and M. Niknam Shahrak, Polyhedron, 2020, 178, 114338 CrossRef CAS.
- M. Kattula, K. Ponnuru, L. Zhu, W. Jia, H. Lin and E. P. Furlani, Sci. Rep., 2015, 5, 1–9 Search PubMed.
- T. Li, Y. Pan, K. V. Peinemann and Z. Lai, J. Membr. Sci., 2013, 425–426, 235–242 CrossRef CAS.
- J. Sánchez-Laínez, M. Ballester-Catalán, E. Javierre-Ortín, C. Téllez and J. Coronas, Dalton Trans., 2020, 49, 2905–2913 RSC.
- A. Selomon, L. Martínez-Izquierdo, M. Malankowska, C. Téllez and J. Coronas, Energy Fuels, 2021, 35, 17085–17102 CrossRef.
- J. P. Sheth, J. Xu and G. L. Wilkes, Polymer, 2003, 44, 743–756 CrossRef CAS.
- T. Yoshida, T. Nakane, M. Uchida and Y. Kaneko, Int. J. Solids Struct., 2022, 239–240, 111419 CrossRef CAS.
- H. Rabiee, A. Ghadimi and T. Mohammadi, J. Membr. Sci., 2015, 476, 286–302 CrossRef CAS.
- Q. Luo and E. Pentzer, ACS Appl. Mater. Interfaces, 2020, 12, 5169–5176 CrossRef CAS PubMed.
- K. Friess, J. C. Jansen, F. Bazzarelli, P. Izák, V. Jarmarová, M. Kačírková, J. Schauer, G. Clarizia and P. Bernardo, J. Membr. Sci., 2012, 415–416, 801–809 CrossRef CAS.
- A. Husna, I. Hossain, I. Jeong and T. H. Kim, Polymers, 2022, 14, 655 CrossRef CAS PubMed.
- K. M. Gupta, Z. Qiao, K. Zhang and J. Jiang, ACS Appl. Mater. Interfaces, 2016, 8, 13392–13399 CrossRef CAS PubMed.
- S. Gadipelli, W. Travis, W. Zhou and Z. Guo, Energy Environ. Sci., 2014, 7, 2232–2238 RSC.
- D. Liu, J. Gu, Q. Liu, Y. Tan, Z. Li, W. Zhang, Y. Su, W. Li, A. Cui, C. Gu and D. Zhang, Adv. Mater., 2014, 26, 1229–1234 CrossRef CAS PubMed.
- L. Martínez-Izquierdo, M. Malankowska, J. Sánchez-Laínez, C. Téllez and J. Coronas, R. Soc. Open Sci., 2019, 6, 190866 CrossRef PubMed.
- A. Ghadimi, M. Amirilargani, T. Mohammadi, N. Kasiri and B. Sadatnia, J. Membr. Sci., 2014, 458, 14–26 CrossRef CAS.
- A. Tena, S. Shishatskiy and V. Filiz, RSC Adv., 2015, 5, 22310 RSC.
- J. H. Kim, Y. Ha and Y. M. Lee, J. Membr. Sci., 2001, 190, 179–193 CrossRef CAS.
- M. M. Rahman, V. Filiz, S. Shishatskiy, C. Abetz, S. Neumann, S. Bolmer, M. M. Khan and V. Abetz, J. Membr. Sci., 2013, 437, 286–297 CrossRef CAS.
- E. Tocci, A. Gugliuzza, L. De Lorenzo, M. Macchione, G. De Luca and E. Drioli, J. Membr. Sci., 2008, 323, 316–327 CrossRef CAS.
- S. Feng, J. Ren, D. Zhao, H. Li, K. Hua, X. Li and M. Deng, J. Energy Chem., 2019, 28, 39–45 CrossRef.
- D. Bakhtin, S. Bazhenov, V. Polevaya, E. Grushevenko, S. Makaev, G. Karpacheva, V. Volkov and A. Volkov, Membr, 2020, 10, 419 CrossRef CAS PubMed.
- H. Z. Chen, Z. Thong, P. Li and T. S. Chung, Int. J. Hydrogen Energy, 2014, 39, 5043–5053 CrossRef CAS.
- S. Lee, S. C. Park, T. Y. Kim, S. W. Kang and Y. S. Kang, J. Membr. Sci., 2018, 548, 358–362 CrossRef CAS.
- J. E. Shin, S. K. Lee, Y. H. Cho and H. B. Park, J. Membr. Sci., 2019, 572, 300–308 CrossRef CAS.
- F. Pardo, G. Zarca and A. Urtiaga, J. Membr. Sci., 2021, 618, 118744 CrossRef CAS.
- A. R. Nabais, L. A. Neves and L. C. Tomé, ACS Appl. Polym. Mater., 2021, 4, 3098–3119 CrossRef.
- P. D. Sutrisna, J. Hou, H. Li, Y. Zhang and V. Chen, J. Membr. Sci., 2017, 524, 266–279 CrossRef CAS.
- S. Y. Rhyu and S. W. Kang, J. Ind. Eng. Chem., 2021, 103, 216–221 CrossRef CAS.
- W. Fam, J. Mansouri, H. Li and V. Chen, J. Membr. Sci., 2017, 537, 54–68 CrossRef CAS.
- Z. De Dai, L. Bai, K. N. Hval, X. P. Zhang, S. J. Zhang and L. Y. Deng, Sci. China: Chem., 2016, 59, 538–546 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta03958c |
| This journal is © The Royal Society of Chemistry 2022 |