Accelerating the dissolution kinetics of iodine with a cosolvent for a high-current zinc–iodine flow battery†
Abstract
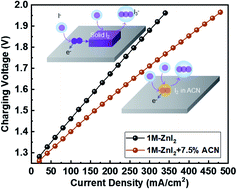
The high reduction potential and the abundance of iodine have prompted its use in the positive electrolytes of aqueous flow batteries, where the transition between highly soluble iodide (I−) and triiodide (I3−) may give rise to superior rate-performance. Yet, the operating currents of Zn–I2 flow batteries remain low, for which the elusive kinetics of the poorly soluble intermediate, iodine (I2), is responsible. With cyclic voltammetry and chronoamperometry, we confirm that solid iodine passivates the carbon electrode, leading to an oscillating current even in a highly concentrated I− solution. UV-Vis spectroscopy and an interface-controlled dissolution model are used to measure the rate constant of the transition from I2 to I3− to be ∼10−6 mol (cm−2 s−1), which agrees with the steady-state current revealed by chronoamperometry and corroborates the current-limiting kinetics. To boost the kinetics, we screen common organic solvents for a cosolvent that can better solvate I2 and find that the addition of 7.5 vol% acetonitrile increases the rate constant by an order of magnitude and the current by more than six times. Calculations using density functional theory reveal the role of the cosolvent in weakening the interaction between iodine and the electrode surface. When employed in a Zn–I2 flow battery, the cosolvent enables a high charging current of over 100 mA cm−2, which is stable for more than 170 cycles, and is promising for high-performance energy storage at an affordable cost.


 Please wait while we load your content...
Please wait while we load your content...