Open Access Article
Open Access ArticleEffect of structure on oxygen diffusivity in layered oxides: a combined theoretical and experimental study†
ChangSub
Kim‡
 ab,
Kyoung-Won
Park‡
ab,
Kyoung-Won
Park‡
 c,
Dmitri
Kalaev
c,
Dmitri
Kalaev
 a,
Clement
Nicollet
a,
Clement
Nicollet
 ad and
Harry L.
Tuller
ad and
Harry L.
Tuller
 *a
*a
aDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: tuller@mit.edu
bJet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91109, USA
cMaterials Architecturing Research Center, Korea Institute of Science and Technology, 5 Hwarang-ro 14-gil Seongbuk-gu, Seoul 02792, Republic of Korea
dUniversité de Nantes, CNRS, Institut des Matériaux Jean Rouxel, IMN, Nantes, France
First published on 3rd June 2022
Abstract
Electrochemical systems based on oxide ion conduction such as solid oxide fuel cells (SOFCs) and solid oxide electrolysis cells (SOECs) provide efficient means of converting energy between chemical and electrical forms, integral to a hydrogen-based energy society. Fundamental understanding of oxygen diffusivity in solids – which can depend on defect type, concentration, as well as migration barrier – is critical in developing their key components: electrolytes and mixed ionic-electronic conducting electrodes. It has been speculated that crystal structure affects the key three factors impacting oxygen diffusion, but no such experiments have been done, without changing cation chemistries, to the best of the authors' knowledge. In this work, oxygen diffusivities in two different but related structures of lanthanum cuprate (La2CuO4+δ) thin films were measured through oxygen isotope exchange experiments and secondary ion mass spectrometry (SIMS) at multiple temperatures. We find that while oxygen diffusion is faster in a T-structured layered cuprate, it nevertheless exhibits a higher activation energy as compared to a T′-structured layered cuprate of the same cation chemistry. This seemingly contradictory phenomenon is explained by a lower oxygen defect formation energy (thus higher oxygen defect concentration) but higher defect migration barrier in the T-structure as calculated by density functional theory, offering insight towards structural design of SOFC and SOEC electrolyte and electrode materials for different target temperatures.
1. Introduction
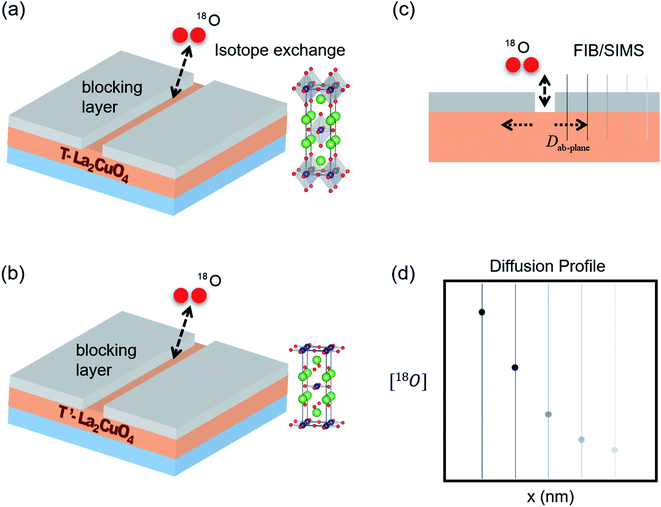
Atomic and ionic diffusion in solids has received growing interest over the past few decades as solid state electrochemical systems such as batteries and fuel cells have become efficient and effective means of generating and storing electrical energy derived from chemical energy.1–6 Diffusion in solids is of particular interest in solid state batteries (lithium ions)7,8 and solid oxide fuel cells (oxygen ions),9–11 as higher diffusivity correlates with higher electrolyte and electrode performances. In the case of oxygen diffusion, there exist two types of oxygen defects – oxygen vacancies and interstitials – that can support oxygen diffusivity. Oxygen ions adjacent to vacant sites enable oxygen ions to hop from normally occupied to adjacent unoccupied sites in the lattice, while interstitials can diffuse via two different mechanisms, viz., via interstitial (from normally occupied to a nearby empty interstitial site), and interstitialcy (interstitial ion displacing a normally occupied ion into an adjacent empty interstitial site) mechanisims.12 Unlike fluorite and perovskite oxides, which can normally only accommodate oxygen vacancies (UO2+x is an exception13), layered oxides can accommodate both types of defects,14–17 thus offering an advantage in their ability to operate at high oxygen partial pressures via oxygen interstitial/interstitialcy diffusion, and at low oxygen partial pressures via oxygen vacancy diffusion.A common form of layered oxide is A2B1O4 – also known as the 214 phase (term n = 1 of the Ruddlesden–Popper series An+1BnO3n+1) – including nickelates, cobaltates, and cuprates in which Ni, Co, and Cu occupy the B-site, respectively. Nickelate and cobaltate 214 phases exhibit the K2NiF4, or T structure, with alternating stacks of rock-salt AO- and perovskite ABO3-layers. Because copper can accommodate three different oxygen coordinations, as shown in Fig. S1,† three different crystal structures exist for the 214 cuprates. For layered cuprates with large A-site cations, such as lanthanum or strontium, copper is octahedrally coordinated by six oxygen atoms (T-A2CuO4). With small A-site cations, such as praseodymium or cerium, copper forms square-planar coordination (T′-A2CuO4), and in between the two phases there is the T* structure, a hybrid of T and T′, in which copper forms pyramidal coordination blocks with oxygen.18 These three structures may share similar chemistries, but show very distinct characteristics. For example, T′-structured Pr2CuO4 has an extremely low oxygen diffusivity of 7.2 × 10−13 cm2 s−1 at 700 °C.19 The incorporation of the larger Sr2+ cations on the A-site leads to the T* structure, as in Pr1.6Sr0.4CuO4, which exhibits a significantly higher oxygen diffusivity of 6.7 × 10−10 cm2 s−1 at 700 °C.20 T-structured La2CuO4 exhibits an even higher oxygen diffusion coefficient D* of around 1 × 10−8 cm2 s−1.21 However, it is unclear whether the difference in diffusivity comes from the crystal structure, since both the cation chemistry and size are changing. Increasing acceptor/donor concentrations will likely lead to larger numbers of oxygen vacancies/interstitials. Different diffusion mechanisms also affect how fast oxygen can migrate. Oxygen diffusion in Pr2CuO4 or T-La2CuO4 is believed to be through oxygen interstitialcy mechanism, while in acceptor-doped Pr1.6Sr0.4CuO4, diffusion is via the oxygen vacancy mechanism. Different cation sizes can affect local charge distribution, lattice parameter and lattice strain, thereby affecting diffusivity. There is no direct comparison of oxygen diffusivities among these structures as each cation chemistry is stable in only one structure. This study aims to answer the question of how different structures of identical cation chemistry affect diffusion and investigate underlying mechanisms by combining experimental (structural control and oxygen isotope exchange) and theoretical (density functional theory) methods.
2. Experimental methods
2.1 Thin film preparation
T and T′-structured La2CuO4, thin films of ∼30 nm thickness were grown by pulsed laser deposition (PLD). After loading single crystal LaAlO3 (LAO) substrates (MTI Corporation) and a bulk target of desired composition (refer to Experimental section in Kim, et al.17), the PLD chamber was pumped down to a base pressure of 3 × 10−6 Torr, and then the substrate was heated to 600 °C. A Coherent COMPex Pro 205 KrF eximer laser of 248 nm wavelength with 260 mJ per pulse at 1 Hz was used to ablate the bulk targets. The surface of each target was pre-ablated with 300–3000 pulses before every deposition. The chamber was maintained at 10 mTorr oxygen pressure during deposition.2.2 Structural characterization
2.3 Oxygen diffusivity measurement
3. Computational methods
3.1 Bulk T- and T′-structures
We modeled bulk T- and T′-structured La2CuO4 (space group = I4/mmm) using a 2 × 2 × 2 supercell composed of 112 atoms with DFT calculations. DFT calculations were performed using VASP22,23 with projected augmented wave (PAW) pseudopotentials from the VASP database and generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE).24 An energy cutoff of 700 eV and a 3 × 3 × 1 Monkhorst–Pack k-point mesh were used after convergence tests. Atom positions were relaxed until all forces were less than 0.005 eV Å−1. Lattice constants of La2CuO4 used in this study are a = b = 3.803 Å and c = 13.15 Å for the T-structure and a = b = 4.005 Å and c = 12.55 Å for the T′-structure.18,25 The probable strain and its effect on defect formation energies by the lattice mismatch between T- and T′-structured La2CuO4 thin films and LaAlO3 substrates are so small that they were not taken into account in this study (explained in more detail in the ESI Fig. S3 and S4†).3.2 Oxygen defect formation energies
To calculate the change in enthalpy when oxygen vacancies or interstitials are generated in La2CuO4, O atoms were removed from or added to the perfect La2CuO4 supercells, and then relaxed as done for the perfect La2CuO4 supercell. The defect formation or generation energy in the field of computational chemistry – when an oxygen defect of charge q is inserted in bulk La2CuO4 is defined as: | (1) |
To describe the defects in experimentally prepared T- and T′-La2CuO4,31,32 we chose single and paired oxygen defects in eight unit cells of T- and T′-La2CuO4±δ (i.e., δ = 1/64 and 1/32). Oxygen defects in T- and T′-La2CuO4±δ were created by removing/adding an oxygen atom in the perfect T- and T′-La2CuO4 structures. The formation energy of each oxygen defect was calculated relative to the perfect LCO crystal, using eqn (1). For examination of defect pairs in T- and T′-La2CuO4±δ, an additional oxygen atom was removed/added from the previous LCO structures with a single oxygen defect. We named the oxygen defect pairs ‘VO2+–VO2+ pairs’ and ‘IO2−–IO2− pairs’. Their defect formation energies were calculated relative to the previous states with single defects using eqn (1), reflecting how much energy is required to create additional oxygen defects from the LCO structures with a single defect in eight unit cells.
3.3 Activation energy for diffusion
The nudged elastic band (NEB) routine33–35 along with the climbing image method34 implemented in VASP, was used with DFT calculations to predict the lowest energy pathway for oxygen diffusion in T- and T′-LCO. The initial and final configurations of diffusion pathways were optimized as done for the relaxation of the structures containing doubly charged defects. Eight images of intermediate atomic configurations were generated with constant spacing between the initial and final configurations. A spring constant of 5.0 eV Å−2 and energy cutoff of 700 eV were used. The ionic relaxations were conducted until all forces were less than 0.005 eV Å−1, and the electronic convergence was terminated at an energy difference of 10−4 eV.4. Results and discussion
4.1 Structural characterization
XRD spectra of La2CuO4 (LCO) thin films grown by PLD on single crystal LaAlO3 (100) substrates with and without seed layer (deposited between substrate and desired film) are shown in Fig. 2. LCO grows as a single-phase T-structure on LaAlO3 without seed layer, but forms a T′-structure when deposited on top of a thin seed layer of La1.85Ce0.15CuO4 (LCCO15). Both films are grown c-axis oriented perpendicular to the substrates.T- and T′-structured LCO thin films without and with the LCCO15 seed layer on LaAlO3 substrates were further investigated by STEM-HAADF and -BF images, as shown in Fig. 3. As can be seen in Fig. 3(a) and S3,† the T-structured LCO thin film grows epitaxially on the LaAlO3(001) substrate and perpendicular to the substrate (parallel to c-axis of the LCO film), which agrees with the results from HRXRD analyses in Fig. 2. The T′-structured LCO film grown on the LCCO15 seed layer/LaAlO3 substrate (Fig. 3(b)) is not as epitaxial compared to the T-LCO film without seed layer, presumably due to lattice mismatch (refer to ‘strain in LCO thin film’ section in ESI†). However, the film is highly crystalline with near-uniform structure and with preferred orientation (c-axis perpendicular to the substrate as analyzed with respect to the locations of La, Cu and O atoms), agreeing with the results from HRXRD in Fig. 2.
The difference in the positions of some oxygen atoms between T- and T′-LCO are clearly observed in the STEM-BF images of Fig. 3; the STEM-BF image of oxygen atoms in apical sites in T-LCO shows brighter line contrast along a- or b-axis of the thin film (denoted with blue dotted lines in Fig. 3(a)) than that of oxygen atoms in equatorial sites, while T′-LCO shows little difference in the STEM-BF contrast of oxygen atoms between O-and CuO2-planes as can be seen in Fig. 3(b). This contrast difference in T- and T′-LCO confirms that T- and T′-LCO thin films form on LAO(001) substrates without and with LCCO15 seed layer.
4.2 Oxygen diffusivities of T- and T′-La2CuO4
The oxygen diffusivities of both T- and T′-LCO thin films are studied to examine the effect of structure on diffusivity with films of the same cation chemistry but different structures. The two structures of LCO are prepared on insulating LAO substrates with and without a LCCO15 seed layer as described in Section 4.1. Cation diffusion is so slow for heavy atoms such as La and Ce that studies have been conducted at 900 °C and above .36–39 By extrapolating the diffusivity temperature dependence down to 600 °C, D of La and Ce comes out to be less than 10−24 cm2 s−1. The highest temperature our samples experienced was at 600 °C for less than two hours (including deposition and isotope exchange experiments), and total diffusion length, comes out to be less than 1 Å. Therefore, it is valid to assume that there is not sufficient Ce diffusion from the seed layer (∼1.5 nm thick) to LCO (∼30 nm thick) to affect oxygen diffusion behavior.
comes out to be less than 1 Å. Therefore, it is valid to assume that there is not sufficient Ce diffusion from the seed layer (∼1.5 nm thick) to LCO (∼30 nm thick) to affect oxygen diffusion behavior.
Oxygen diffusion in the ab-plane is faster than along the c-axis as evidenced in the isostructural nickelates,40,41 and will dominate the performance in polycrystalline bulk materials used for practical purposes. Therefore, diffusion in the ab-plane for the two structures is compared with films oriented c-axis perpendicular to the substrate. After the 18O isotope exchange is completed, the isotope profiles are obtained by SIMS as described in experimental methods and Fig. 1. Oxygen isotope concentration–distance profiles for T- and T′-LCO are plotted in Fig. 4(a) and (b). The 18O isotope density in both thin films decreases along the direction of in-plane diffusion. The distribution of the 18O isotope in the T-LCO thin film extends further from the origin than in the T′-LCO thin film over the same period of time, indicating that the 18O isotope exhibited a higher diffusivity in T-LCO than in T′-LCO.
 | ||
| Fig. 4 18O isotope concentration profiles measured by ToF-SIMS after oxygen isotope exchange for one hour at aO2 = 0.5 atm and 600 °C for (a) T- and (b) T′-La2CuO4 thin films. | ||
 | ||
Fig. 5 Oxygen tracer diffusion coefficients along the ab-plane  for T- and T′-LCO thin films at aO2 = 0.5 atm as a function of inverse temperature (1/T). for T- and T′-LCO thin films at aO2 = 0.5 atm as a function of inverse temperature (1/T). | ||
Values for  (oxygen diffusion coefficient along ab-plane) are extracted by fitting the data with the aid of Crank's solution42 for diffusion in a semi-infinite medium. The fits to the data in Fig. 4 resulted in
(oxygen diffusion coefficient along ab-plane) are extracted by fitting the data with the aid of Crank's solution42 for diffusion in a semi-infinite medium. The fits to the data in Fig. 4 resulted in  = 1.06 × 10−8 cm2 s−1 for T-LCO and
= 1.06 × 10−8 cm2 s−1 for T-LCO and  = 1.30 × 10−9 cm2 s−1 for T′-LCO at 600 °C showing that the oxygen diffusivity for the T-structure is approximately ten times higher than that in the T′-structure.
= 1.30 × 10−9 cm2 s−1 for T′-LCO at 600 °C showing that the oxygen diffusivity for the T-structure is approximately ten times higher than that in the T′-structure.
Oxygen tracer diffusion experiments were performed at two additional temperatures, 500 and 550 °C, with the derived diffusion coefficients plotted in Fig. 4. The temperature dependence of diffusivity can be expressed as
 | (2) |
4.3 Oxygen defect formation energies
In oxygen-diffusion mediated materials, a higher oxygen diffusion coefficient is generally correlated with a lower activation energy for diffusion (Ea).43 However, we observe that while the T-structured LCO shows faster oxygen diffusion, it nevertheless exhibits a higher activation barrier compared to T′-structured LCO. In an attempt to explain this unexpected relationship, we examine the respective defect chemistries of T- and T′-structured LCO, utilizing DFT calculations (described in Computational methods section) to identify the dominant type(s) of oxygen defects contributing to diffusion and their relative concentrations in the two structures.
is generally correlated with a lower activation energy for diffusion (Ea).43 However, we observe that while the T-structured LCO shows faster oxygen diffusion, it nevertheless exhibits a higher activation barrier compared to T′-structured LCO. In an attempt to explain this unexpected relationship, we examine the respective defect chemistries of T- and T′-structured LCO, utilizing DFT calculations (described in Computational methods section) to identify the dominant type(s) of oxygen defects contributing to diffusion and their relative concentrations in the two structures.
 is 7.95 × 1015 and 2.01 × 107 for doubly-charged oxygen interstitials in T- and T′-LCO, respectively.
is 7.95 × 1015 and 2.01 × 107 for doubly-charged oxygen interstitials in T- and T′-LCO, respectively.
 | ||
| Fig. 7 Images: atomic configurations of T-LCO showing the locations of (a) VO2+–VO2+ pairs and (b) IO2−–IO2− pairs in T-LCO. Green, blue, red circles denote La, Cu, O atoms, respectively. ‘E’ and ‘I’ in gray and yellow circles denote the first oxygen vacancy existing on equatorial oxygen site (1st VO2+) and first oxygen interstitial existing in the most possible/stable sites (1st IO2−) in T-LCO according to Fig. 6(a), respectively. ‘I’ and ‘II’ in pink circles indicate the 2nd defect position at the 1st and 2nd nearest neighbors from the 1st defect. Yellow and pink circles on the right side of (b) denote 1st IO2− and 2nd IO2−, respectively. Tables: defect formation energy (Ef) of (a) VO2+–VO2+ pairs and (b) IO2−–IO2− pairs in T-LCO, with respect to the 2nd defect location. Unit is eV per defect. When the 2nd IO2− exists in the 2nd nearest neighbor (position II in (b)), the T-LCO structure becomes unstable and the 2nd IO2− moves to position I. Therefore, the formation energy of the 2nd IO2− at position II could not be calculated. | ||
 | ||
| Fig. 8 Images: atomic configurations of T′-LCO showing the locations of (a) VO2+–VO2+ pairs and (b) IO2−–IO2− pairs in T′-LCO. Green, blue, red circles denote La, Cu, O atoms, respectively. ‘O’ and ‘I’ in gray and yellow circles denote oxygen vacancy in O-plane and oxygen interstitial in the most stable interstitial site in T′-LCO (according to Fig. 6(b)), respectively. Numbers ‘1’ through ‘4’ in pink circles indicate the 2nd defect position relative to the 1st defect. Yellow and pink circles on the right side of (b) denote 1st IO2− and 2nd IO2−, respectively. Tables: defect formation energy (Ef) of (a) VO2+–VO2+ pairs and (b) IO2−–IO2− pairs in T′-LCO, with respect to the 2nd defect location. Unit is eV per defect. | ||
As summarized in the tables in Fig. 7, T-LCO strongly favors a second oxygen interstitial in the 1st nearest neighbor position (position I in Fig. 7(b)) to form a pair, given the highly negative formation energy of IO2−–IO2− pair. Oxygen vacancy pairs, on the other hand, hardly form in T-LCO, regardless of the locations of the defect pairs. This result is in accordance with the formation energy of a single oxygen defect shown in Fig. 6(a); T-LCO thermodynamically prefers to form a high concentration of interstitials while incapable of accommodating oxygen vacancies.
For T′-LCO, both VO2+–VO2+ (Fig. 8(a)) and IO2−–IO2− pairs (Fig. 8(b)) have negative formation energies, and therefore local regions with both oxygen vacancy and interstitial pairs can exist. Among several configurations with different neighbors, the 2nd nearest neighbor (position 1 in Fig. 8) is the most energetically favorable for vacancy defects. For interstitial defects, the 1st nearest neighbor pair is the most energetically favorable. Note that this is only considering oxygen interstitials; oxygen atoms on O- and CuO2-planes as well as lanthanum and copper atoms are closer than the first nearest interstitial.
4.4 Oxygen diffusion path
To find the minimum energy path (MEP) for oxygen diffusion along the ab-plane in T- and T′-structured La2CuO4, diffusion energy barriers were calculated using NEB methods as explained in Computational methods for every possible migration pathway taken by oxygen vacancies or interstitials in the structures. For the NEB calculations, only doubly charged oxygen interstitials and vacancies were considered given that they are thermodynamically favored to be generated, according to the defect deformation energies reported in Section 4.3. The diffusion pathways along the ab-plane were considered only when oxygen point defects migrate via first- or second-nearest neighbor sites, as indicated in Fig. 9–12. Some examples of energy landscapes calculated along diffusion coordinates of defects in T- and T′-structured La2CuO4 were shown in Fig. S7.† | ||
| Fig. 10 (a) Two (A and B) diffusion pathways of doubly charged oxygen interstitials (marked with “I” indicated by yellow circle) along ab-plane in T-LCO, when an additional oxygen interstitial (2nd IO2−) exists in the nearest neighbor (marked with “I” in pink circle). Paths A and B are interstitialcy migration pathways. (b) Atomic configurations of T-LCO perpendicular to ab-plane (oxygen diffusion plane) showing the paths A and B, when 2nd IO2− is present in position I (refer to Fig. 7). Yellow, pink and cyan circles denote 1st IO2−, 2nd IO2−, and a lattice oxygen atom participating in the migration. Green, blue, red circles are La, Cu, O atoms, respectively, that do not directly participate in the migration. Table: diffusion energy barriers of doubly charged oxygen interstitials along paths A and B shown in figures (a) and (b). | ||
 | ||
| Fig. 12 (a) Left: diffusion pathway 2′ of doubly charged oxygen vacancy (marked with “O” in yellow circle, 1st VO2+) on O-plane in T′-LCO, when an additional oxygen vacancy (2nd VO2+) exists in close distances (positions 1 to 4 in pink circles). Table: diffusion energy barriers of oxygen via 1st VO2+ movement along path 2′, when there are 2nd VO2+ in positions 1–4 (refer to Fig. 8(a)). (b) Left: diffusion pathway C′ of doubly charged oxygen interstitials (marked with “I” in yellow circle, 1st IO2−) in T′-LCO, when an additional oxygen interstitial (2nd IO2−) exists in the first and second nearest neighbors (positions 1 and 2 in pink circles). Table: diffusion energy barriers of oxygen via 1st IO2− movement along path C′, when 2nd IO2− exists in position 2 (refer to Fig. 8(b)). Bottom right: atomic configurations of T′-LCO perpendicular to ab-plane (oxygen diffusion plane) showing the path C′, when 2nd IO2− is present in position 2 (refer to Fig. 8(b)). Yellow, and pink circles denote 1st IO2− and 2nd IO2−, respectively. Green, blue, red circles are La, Cu, O atoms, respectively, that do not directly participate in the migration. | ||
In the case of interstitial atoms (Fig. 11(b)), the direct interstitial mechanism along the ab-plane (path C′) shows a minimum energy barrier for diffusion (1.94 eV), unlike the interstitialcy mechanism in T-La2CuO4 (path A in Fig. 9). However, this value is also much higher than the experimental value.
For oxygen vacancy pairs (Fig. 12(a)), the energy barrier for oxygen movement via path 2′ does not show a discernible change in comparison with that of a single vacancy (Fig. 11(a)), regardless of the relative position of VO2+–VO2+ pairs (position 1 to 4 in Fig. 12(a)). Whereas, oxygen interstitial pairs (position 2 in Fig. 12(b)) show a decrease in migration barrier for diffusion from a single, unpaired interstitial (1.94 → 1.06 eV) along the direct interstitial diffusion pathway (path C′). This reduced value agrees well with the experimentally derived one of 1.03 eV (Fig. 4). Therefore, we conclude that the oxygen diffusion in T′-LCO is via direct interstitial mechanism with the help of neighboring oxygen interstitials resulting in a lower activation energy for diffusion as observed in the experiment.
4.5. Summary
The defect formation energies (Ef) and migration energies (Em) calculated by DFT, as well as pre-exponential terms (D0) and activation energies (Ea,D) of diffusion from oxygen isotope exchange experiments for T and T′ structures of LCO are summarized in Table 1. Oxygen vacancy formation energies for T-La2CuO4 are positive (see Fig. 6), and therefore are excluded from the table. The oxygen interstitial formation energy for the T-structure is found to be highly negative (−2.83 eV). This implies the ready formation of a high concentration of oxygen interstitials already at reduced temperatures, and thus oxygen diffusion is predicted to be limited only by the migration barrier. This is confirmed by the agreement between the activation energy of diffusion 1.79 ± 0.03 eV from the isotope exchange experiment and the calculated migration energy of 1.81 eV. It should be noted that this is unlike the case of traditional mixed ionic and electronic conducting (MIEC) oxides in which the overall diffusion activation energy includes a contribution from thermally activated defect formation, given a low initial defect concentration at reduced temperatures and increase in oxygen vacancies at higher temperatures.| Structure | T | T′ | |
| Defect type | I O 2− | VO2+ | I O 2− |
| E f (eV), DFT | −2.83 | −1.2 | −1.3 |
| Pair Ef (eV), DFT | −2.07 | −0.53 | −0.9 |
| exp(−Ef/kBT), T = 897 K | 7.95 × 1015 | 5.52 × 106 | 2.01 × 107 |
| D 0 (cm2 s−1), experiment | 2.21 × 102 | 1.07 × 10−3 | |
| Diffusion mechanism | Interstitialcy | Vacancy | Direct interstitial |
| E m (eV), DFT | 1.81 | 2.09 | 1.94 |
| Pair Em (eV), DFT | 1.96 | 1.92 | 1.06 |
| E a,D (eV), experiment | 1.79 ± 0.03 | 1.03 ± 0.24 | |
Oxygen vacancy formation energies for T′-La2CuO4 are negative and comparable to that of oxygen interstitials. However, since the vacancy migration energies are significantly higher than that of the pair-wise interstitial diffusion, the net diffusion is dominated by oxygen interstitials. This, again, is confirmed by comparing the migration energy of interstitial pairs (1.06 eV) as calculated by DFT and the activation energy of diffusion 1.03 ± 0.24 eV as obtained from the experiment. Because of much lower interstitial formation energy for T-LCO, the amount of oxygen interstitials is likely much greater in T-LCO compared to T′-LCO, thus much larger pre-exponential term in diffusivity.
In summary, oxygen diffusion in T-LCO is dominated by large concentration of oxygen interstitials migrating via interstitialcy mechanism, while T′-LCO has several orders of magnitude lower concentrations of oxygen defects and oxygen diffusion is via direct interstitial mechanism as interstitial pairs with significantly lower migration barrier compared to T-LCO. For both structures, thermal activation is determined solely by the corresponding migration energies. This is consistent with our earlier findings of surprisingly low reduction/oxidation enthalpies that result in defect concentrations being insensitive to temperature excursions at a given aO2.17,32
5. Conclusions
The effect of structure on oxygen diffusion was successfully separated from those induced by variations in cation chemistry by successfully growing two different structures of La2CuO4 in thin film form with the aid of buffer layers. Oxygen diffusivity as derived from isotope exchange studies with the aid of SIMS profiling is faster in T-than in T′-structured La2CuO4, while the activation energy of diffusion is surprisingly lower for the T′-structure. Oxygen defect formation energies and migration energies were calculated by DFT for both types of oxygen defects in the T and T′ structures. Oxygen interstitials are the dominant defect species for both structures. Oxygen diffusion is through interstitialcy mechanism in T-LCO, but is through direct interstitial mechanism in pairs in T′-LCO. The magnitude of diffusivity is dominated by the density of available mobile defects, and is therefore higher in the T-structure which exhibits considerably lower defect formation energies. The activation energies for diffusion as derived from oxygen isotope exchange experiments are directly correlated with the activation energies of migration calculated by DFT, explaining the lower activation energy for diffusion in the T′-structure. This provides an insight into creating models for diffusion mechanisms in layered structures with multiple defect species by combining experimental and computational approaches.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is based on work funded by the Skolkovo Institute of Science and Technology (Skoltech), with program name “Center for Research, Education and Innovation for Electrochemical Energy Storage” under contract number 186-MRA. The computational research was supported by the International Research & Development Program of the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (2019K1A4A7A02102530), and the National Supercomputing Center with supercomputing resources including technical support (KSC-2020-CRE-0246). X-ray diffraction scans of the samples were conducted in the MRSEC Shared Experimental Facilities at MIT, supported by the National Science Foundation under award number DMR-1419807. STEM imaging was performed by the Advanced Analysis Center at the Korea Institute of Science and Technology.References
- M. Winter and R. J. Brodd, What Are Batteries, Fuel Cells, and Supercapacitors?, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- E. Kendrick and P. Slater, Battery and solid oxide fuel cell materials, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2012, 108, 424 RSC.
- L. Bi, E. Fabbri, Z. Sun and E. Traversa, A novel ionic diffusion strategy to fabricate high-performance anode-supported solid oxidefuel cells (SOFCs) with proton-conducting Y-doped BaZrO3 films, Energy Environ. Sci., 2011, 4, 409–412 RSC.
- W. Li, Y. Yang, G. Zhang and Y.-W. Zhang, Ultrafast and Directional Diffusion of Lithium in Phosphorene for High-Performance Lithium-Ion Battery, Nano Lett., 2015, 15, 1691–1697 CrossRef CAS PubMed.
- H. Li, N. L. Okamoto, T. Hatakeyama, Y. Kumagai, F. Oba and T. Ichitsubo, Fast Diffusion of Multivalent Ions Facilitated by Concerted Interactions in Dual-Ion Battery Systems, Adv. Energy Mater., 2018, 8, 1801475 CrossRef.
- Y. Xia, Z. Jin, H. Wang, Z. Gong, H. Lv, R. Peng, W. Liu and L. Bi, A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance, J. Mater. Chem. B, 2019, 7, 16136–16148 RSC.
- A. Bates, S. Mukherjee, N. Schuppert, B. Son, J. G. Kim and S. Park, Modeling and simulation of 2D lithium-ion solid state battery: Effects of materials and geometry on the performance of SSB, Int. J. Energy Res., 2015, 39, 1505–1518 CrossRef CAS.
- Y. Nomura, K. Yamamoto, T. Hirayama, E. Igaki and K. Saitoh, Visualization of Lithium Transfer Resistance in Secondary Particle Cathodes of Bulk-Type Solid-State Batteries, ACS Energy Lett., 2020, 5, 2098–2105 CrossRef CAS.
- A. Chroneos, R. V. Vovk, I. L. Goulatis and L. I. Goulatis, Oxygen transport in perovskite and related oxides: A brief review, J. Alloys Compd., 2010, 494, 190–195 CrossRef CAS.
- A. B. Muñoz-García, A. M. Ritzmann, M. Pavone, J. A. Keith and E. A. Carter, Oxygen Transport in Perovskite-Type Solid Oxide Fuel Cell Materials: Insights from Quantum Mechanics, Acc. Chem. Res., 2014, 47, 3340–3348 CrossRef PubMed.
- F. H. Taylor, J. Buckeridge and C. R. A. Catlow, Defects and Oxide Ion Migration in the Solid Oxide Fuel Cell Cathode Material LaFeO 3, Chem. Mater., 2016, 28, 8210–8220 CrossRef CAS.
- A. Chroneos, B. Yildiz, A. Tarancón, D. Parfitt and J. A. Kilner, Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: mechanistic insights from atomistic simulations, Energy Environ. Sci., 2011, 4, 2774 RSC.
- T. G. Stratton and H. L. Tuller, Thermodynamic and transport studies of mixed oxides. The CeO2–UO2 system, J. Chem. Soc., Faraday Trans. 2, 1987, 83, 1143–1156 RSC.
- D. Lee and H. Lee, Controlling Oxygen Mobility in Ruddlesden–Popper Oxides, Materials, 2017, 10, 368 CrossRef PubMed.
- K. Mukherjee, Y. Hayamizu, C. S. Kim, L. M. Kolchina, G. N. Mazo, S. Ya. Istomin, S. R. Bishop and H. L. Tuller, Praseodymium Cuprate Thin Film Cathodes for Intermediate Temperature Solid Oxide Fuel Cells: Roles of Doping, Orientation, and Crystal Structure, ACS Appl. Mater. Interfaces, 2016, 8, 34295–34302 CrossRef CAS PubMed.
- C. S. Kim and H. L. Tuller, Fine-tuning of oxygen vacancy and interstitial concentrations in La1.85Ce0.15CuO4+δ by electrical bias, Solid State Ionics, 2018, 320, 233–238 CrossRef CAS.
- C. S. Kim, K.-W. Park and H. L. Tuller, Electrochemically controlled defect chemistry: From oxygen excess to deficiency, Acta Mater., 2021, 211, 116866 CrossRef CAS.
- M. Naito, A. Tsukada, T. Greibe and H. Sato, Phase control in La-214 epitaxial thin films, Proc. SPIE Superconducting and Related Oxides: Physics and Nanoengineering V, 2002, vol. 4811, pp. 140–154 Search PubMed.
- M. S. Kaluzhskikh, S. M. Kazakov, G. N. Mazo, S. Ya. Istomin, E. V. Antipov, A. A. Gippius, Yu. Fedotov, S. I. Bredikhin, Y. Liu, G. Svensson and Z. Shen, High-temperature crystal structure and transport properties of the layered cuprates Ln2CuO4, Ln=Pr, Nd and Sm, J. Solid State Chem., 2011, 184, 698–704 CrossRef CAS.
- G. N. Mazo, S. M. Kazakov, L. M. Kolchina, S. Ya. Istomin, E. V. Antipov, N. V. Lyskov, M. Z. Galin, L. S. Leonova, Yu. S. Fedotov, S. I. Bredikhin, Y. Liu, G. Svensson and Z. Shen, Influence of structural arrangement of R2O2 slabs of layered cuprates on high-temperature properties important for application in IT-SOFC, Solid State Ionics, 2014, 257, 67–74 CrossRef CAS.
- E. Boehm, J.-M. Bassat, M. C. Steil, P. Dordor, F. Mauvy and J.-C. Grenier, Oxygen transport properties of La2Ni1−xCuxO4+δ mixed conducting oxides, Solid State Sci., 2003, 5, 973–981 CrossRef CAS.
- G. Kresse and J. Hafner, Ab initio molecular dynamics for liquid metals, Phys. Rev. B, 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium, Phys. Rev. B, 1994, 49, 14251–14269 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- A. Tsukada, M. Naito and H. Yamamoto, Valence of Ce in T- and T′-La2−xCexCuO4, Phys. C, 2007, 463–465, 64–67 CrossRef CAS.
- J. Meng, L. Zhang, F. Yao, X. Liu, J. Meng and H. Zhang, Density Functional Characterization of the 4f-Relevant Electronic Transitions of Lanthanide-Doped Lu2O 3 Luminescence Materials, ChemPhysChem, 2018, 19, 2947–2953 CrossRef CAS PubMed.
- S. Pesant and M. Côté, DFT + U study of magnetic order in doped La2CuO4 crystals, Phys. Rev. B, 2011, 84, 085104 CrossRef.
- M. Enhessari, M. Shaterian, M. J. Esfahani and M. N. Motaharian, Synthesis, characterization and optical band gap of La2CuO4 nanoparticles, Mater. Sci. Semicond. Process., 2013, 16, 1517–1520 CrossRef CAS.
- S. Zhang and J. Northrup, Chemical potential dependence of defect formation energies in GaAs: Application to Ga self-diffusion, Phys. Rev. Lett., 1991, 67, 2339–2342 CrossRef CAS PubMed.
- C. Freysoldt, B. Grabowski, T. Hickel, J. Neugebauer, G. Kresse, A. Janotti and C. G. Van de Walle, First-principles calculations for point defects in solids, Rev. Mod. Phys., 2014, 86, 253–305 CrossRef.
- H. Kanai, J. Mizusaki, H. Tagawa, S. Hoshiyama, K. Hirano, K. Fujita, M. Tezuka and T. Hashimoto, Defect Chemistry of La2−xSrxCuO4-δ: Oxygen Nonstoichiometry and Thermodynamic Stability, J. Solid State Chem., 1997, 131, 150–159 CrossRef CAS.
- E. J. Opila and H. L. Tuller, Thermogravimetric Analysis and Defect Models of the Oxygen Nonstoichiometry in La2−xSrxCuO4-y, J. Am. Ceram. Soc., 1994, 77, 2727–2737 CrossRef CAS.
- G. Henkelman and H. Jónsson, Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points, J. Chem. Phys., 2000, 113, 9978–9985 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- D. Sheppard and G. Henkelman, Paths to which the nudged elastic band converges, J. Comput. Chem., 2011, 32, 1769–1771 CrossRef CAS PubMed.
- M. Izuki, M. E. Brito, K. Yamaji, H. Kishimoto, D.-H. Cho, T. Shimonosono, T. Horita and H. Yokokawa, Interfacial stability and cation diffusion across the LSCF/GDC interface, J. Power Sources, 2011, 196, 7232–7236 CrossRef CAS.
- V. M. Bekale, A. M. Huntz, C. Legros, G. Sattonnay and F. Jomard, Impurity diffusion of cerium and gadolinium in single- and polycrystalline yttria-stabilized zirconia, Philos. Mag., 2008, 88, 1–19 CrossRef CAS.
- T. Akashi, M. Nanko, T. Maruyama, Y. Shiraishi and J. Tanabe, Solid-State Reaction Kinetics of LaCrO3 from the Oxides and Determination of La3+ Diffusion Coefficient, J. Electrochem. Soc., 1998, 145, 2090–2094 CrossRef CAS.
- S. Beschnitt, T. Zacherle and R. A. De Souza, Computational Study of Cation Diffusion in Ceria, J. Phys. Chem. C, 2015, 119, 27307–27315 CrossRef CAS.
- J. Bassat, Anisotropic ionic transport properties in La2NiO4+δ single crystals, Solid State Ionics, 2004, 167, 341–347 CrossRef CAS.
- M. Burriel, G. Garcia, J. Santiso, J. A. Kilner, R. J. Chater and S. J. Skinner, Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+δ, J. Mater. Chem., 2008, 18, 416–422 RSC.
- J. Crank, The Mathematics of Diffusion, Oxford University Press, 2nd edn., 1980 Search PubMed.
- H. Tuller, in Springer Handbook of Electronic and Photonic Materials, ed. S. Kasap and P. Capper, Springer International Publishing, Cham, 2017, pp. 247–266 Search PubMed.
- W. Xie, Y.-L. Lee, Y. Shao-Horn and D. Morgan, Oxygen Point Defect Chemistry in Ruddlesden–Popper Oxides (La1−xSrx)2MO4±δ (M = Co, Ni, Cu), J. Phys. Chem. Lett., 2016, 7, 1939–1944 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta02580a |
| ‡ Equally contributed. |
| This journal is © The Royal Society of Chemistry 2022 |