New framework of integrated electrocatalysis systems for nitrogen fixation
Abstract
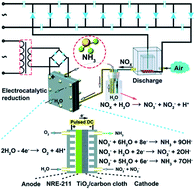
Nitrogen is one of the most essential molecules for daily life, especially as the utilization of nitrogen fertilizers prevents malnutrition. However, the generation of nitrogen fertilizers still relies on conventional chemical methods and exploring novel nitrogen conversion methods is of great significance. The electrochemical fixation method is a highly desirable approach due to its excellent production efficiency and lower environmental pollution. In this paper, we highlight the novel framework of integrated electrocatalysis systems for nitrogen fixation based on nanogenerators, as they are different from the typical nitrogen reduction reaction systems with a continuous high power supply. The novel hybridized and integrated nitrogen fixation system was demonstrated and introduced based on self-powered triboelectric nanogenerators (TENGs) as the power generator. Meanwhile, we also put forward distinct perspectives for enhancing the effectiveness of the hybridized system. This review will present advanced concepts for developing a next-generation nitrogen fixation system by integrating a TENG green energy harvester.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...