Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
MXene-based multifunctional smart fibers for wearable and portable electronics†
Leiqiang
Qin‡
 *a,
Jianxia
Jiang‡
ab,
Lintao
Hou
*a,
Jianxia
Jiang‡
ab,
Lintao
Hou
 c,
Fengling
Zhang
a and
Johanna
Rosen
*a
c,
Fengling
Zhang
a and
Johanna
Rosen
*a
aDepartment of Physics, Chemistry and Biology (IFM), Linköping University, Linköping, SE-58183, Sweden. E-mail: leiqiang.qin@liu.se; johanna.rosen@liu.se
bFlexible Electronics Innovation Institute, Jiangxi Science and Technology Normal University, Nanchang, 330013, China
cGuangzhou Key Laboratory of Vacuum Coating Technologies and New Energy Materials, Physics Department, Jinan University, Guangzhou, 510632, China
First published on 30th May 2022
Abstract
Fiber type devices are promising for applications in wearable and portable electronics. However, scalable fabrication of fiber electrodes with multifunctional performance for use in distinct fields remains challenging. Herein, high performance smart fibers based on Mo1.33C i-MXene nanosheets and poly(3,4-ethylenedioxythiophene):polystyrene sulfonate hybrid paste are fabricated with an easily scalable spinning approach. The hybrid fibers produced by this method can be applied in both high-performance supercapacitors and electrochemical transistors (ECTs). When assembled into a fiber type asymmetric supercapacitor with reduced graphene oxide (rGO) fiber, a capacitance of 105 F g−1 and an energy density of 37 mW h g−1 were reached for a potential window of 1.6 V. The hybrid fiber based ECT shows high transconductance and fast response time. This work demonstrates the potential of i-MXene-based fiber electrodes for multifunctional applications, to aid in the development of the next-generation, high-performance wearable electronic devices.
Introduction
Fiber-type opto-electronic components such as solar cells,1–3 supercapacitors,4 batteries5 and transistors6–8 with nonplanar device architectures have received significant attention for convenient integration into ‘smart’ fabrics. Important recent advances in fiber-type devices have exploited novel active materials. In particular, compared to their bulk counterparts, two-dimensional (2D) nanomaterials with fascinating properties and easily assembled structures in the form of nanoscale frameworks are building blocks for textile devices.9–13 Until now, a growing number of 2D materials have garnered considerable attention for developing into macroscopic fibrous structures, including carbon nanotubes (CNTs),14–16 reduced graphene oxide (rGO),17–21 transition metal dichalcogenides (TMDs),22 and metal oxides.23–25 For example, significant progress has been made in development of one-dimensional (1D) carbon-based fibers prepared from CNTs or graphene oxides (GO). Carbon-based fibers possess outstanding physical and chemical properties, such as excellent electrical and thermal conductivities and high flexibility, mechanical strength, and chemical stabilities, meeting the demands of the next generation of smart electronic devices woven into textiles.26 However, compared to their corresponding film morphology, the properties of carbon-based fibers are significantly reduced. Especially for carbon-based micro-supercapacitors it remains challenging to achieve a high capacitance since the large specific surface area of the nanomaterial is not effectively utilized due to restacking of the nanosheets. In addition, insufficient attention has been paid to development of multifunctional fibers, which could be achieved through rational design by selecting suitable materials or combining two materials with different functionalities.MXenes, as a growing family of 2D transition metal carbides and nitrides with excellent conductivity, mechanical strength, and potential for a wide range of applications, have been extensively explored.27–33 MXenes are typically obtained by selectively etching the A layers in the atomically laminated Mn+1AXn phases,34,35 where M is a transition metal, A is an A-group element, X is C and/or N. In particular, the pseudocapacitive charge storage mechanism and the increased surface area upon delamination of the multilayered MXene into single or few layers facilitates capacitance values superior to carbon materials. In 2017, we reported a new type of quaternary i-MAX phases, (Mo2/3Sc1/3)2AlC and (Mo2/3Y1/3)2AlC, with two different M elements which are in-plane chemically ordered.36 The first in-plane vacancy ordered i-MXene, Mo1.33C, was prepared by selective removal of both the Al and Sc atoms from (Mo2/3Sc1/3)2AlC. The ordering of the vacancies together with a high conductivity enabled Mo1.33C with one of the highest volumetric capacitances (≈1150 F cm−3) reported to date for a film electrode based on a 2D material. Furthermore, by post etching treatment of the electrode, or by combining the i-MXene with conducting polymer poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS), a higher capacitive performance can be achieved for electrodes based on MXene only and composite film electrode architectures, respectively.37,38 However, film electrodes obtained by traditional electrode fabrication methods suffer severe performance degradation for thick films or high rates.
Herein, we present the design and fabrication of flexible and efficient MXene based multifunctional smart fibers through a straightforward and scalable synthetic route using Mo1.33C/PEDOT:PSS hybrid paste where PEDOT:PSS as a conductive binder promotes spinnability and flexibility as well as an increased interlayer spacing between Mo1.33C i-MXene layers (Fig. 1a and b). The as-prepared i-MXene-based fiber is favorable for transport and storage of charge due to its interconnected network architecture which is rich in mesopores. Fiber-type supercapacitors (FSCs) fabricated with these fibers is shown to provide a combination of high capacitance, high rate capabilities and excellent cycling stability. In addition, an asymmetric fiber-type device was constructed by using the Mo1.33C/PEDOT:PSS hybrid fiber and rGO fiber, which can be used both for supercapacitors and electrochemical transistors (ECTs). The resulting asymmetric fiber type supercapacitor (AFSCs) is extremely flexible and can operate in a wide potential window of 1.6 V, with high capacitance, excellent rate and cycling stability, as well as with an improved energy density. Furthermore, the water-based i-MXene ECTs exhibit shorter switching time, higher transconductance and Ion/off ratios, compared to pristine PEDOT:PSS. In this work, the multifunctional smart i-MXene fibers prepared by a scalable method suggest that they are promising candidates for high-performance, portable, flexible, and wearable electronics.
Experimental section
Materials synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was stirred for 5 min and then dropped in 1 M H2SO4 solution. After centrifugation at 4000 rpm for 10 min, the hybrid paste was washed by deionized water 3 times.
1. The mixture was stirred for 5 min and then dropped in 1 M H2SO4 solution. After centrifugation at 4000 rpm for 10 min, the hybrid paste was washed by deionized water 3 times.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and then dried by freeze drying. Based on the prepared fiber, the mass loading of the hybrid fiber electrodes is 24 mg cm−2 and the mass loading of the symmetric device is 36 mg cm−2.
4) and then dried by freeze drying. Based on the prepared fiber, the mass loading of the hybrid fiber electrodes is 24 mg cm−2 and the mass loading of the symmetric device is 36 mg cm−2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–120
000–120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was added to 10 ml deionized water and was then stirred at 85 °C for 10 h until a clear solution was obtained. 1 g of H2SO4 was added to the solution and cooled down to room temperature and then stirred for 2 h.
000) was added to 10 ml deionized water and was then stirred at 85 °C for 10 h until a clear solution was obtained. 1 g of H2SO4 was added to the solution and cooled down to room temperature and then stirred for 2 h.
Characterization
The capacitor performance tests were performed using a VSP potentiostat (BioLogic, France). In the three-electrode configuration, the fiber was used as working electrode, a platinum foil as the counter electrode and Ag/AgCl in 1 M KCl as the reference electrode, using an aqueous solution containing H2SO4 (3 M) as the electrolyte. For the two-electrode symmetrical supercapacitor device, the i-MXene based hybrid fiber as both working electrodes and counter electrode. For AFSCs, the rGO fiber as the working electrodes and the i-MXene based hybrid fiber as counter electrode. The impedance measurements were performed at an open circuit potential with a 5 mV amplitude in a frequency range from 10 mHz to 100 kHz. The electrical characterization of the fiber type ECTs was carried out by means of a Keithley 4200 semiconductor parameter analyzer. The Mo1.33C/PEDOT:PSS hybrid fiber was used as source and drain terminals and the rGO fiber as gate electrode. The transistor channel was defined by the contact area of the gel electrolyte with the source-drain fiber. The morphology of the fibers were carried out using SEM (LEO 1550 Gemini). XRD was carried out on a PANalytical X'Pert diffractometer using Cu Kα radiation (45 kV and 40 mA). Atomic force microscopy (AFM) was performed at ambient conditions (room temperature in a lab) using a Veeco DI Dimension 3100 scanning probe microscope, equipped with nanoscope IV electronics. The measurements were performed in tapping mode using Si tips (PPPNCHR-50 from Nanosensors) with a tip radius of curvature < 7 nm.Results and discussion
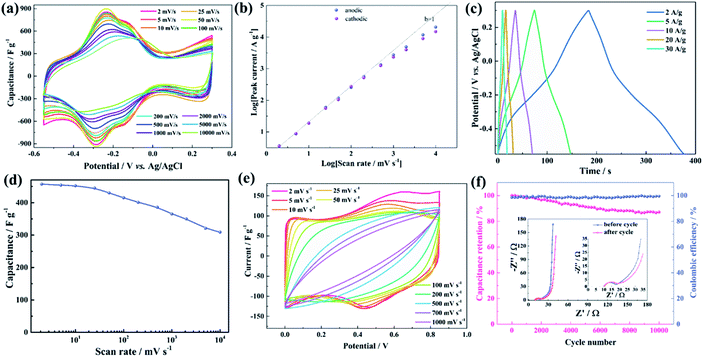
The delaminated Mo1.33C i-MXene was obtained by selectively etching the Al and Sc from the i-MAX phase (Mo2/3Sc1/3)2AlC, as reported previously.36 The successful etching and exfoliation of the i-MAX phase is confirmed by the disappearance of the peak at 2θ = 13.1° (i-MAX phase) and a new peak appearing at 7.2° (i-MXene) in the XRD pattern (Fig. S1†). As shown in the SEM image, the average lateral size of exfoliated Mo1.33C monolayers is around 1.3 μm (Fig. 1c). In addition, from atomic force microscopy (AFM) mapping it is revealed that the thickness of the Mo1.33C monolayers is around 1.2 nm (Fig. 1d), which is as expected and thinner compared to previously reported Ti3C2Tz MXene.29 The Mo1.33C/PEDOT:PSS fiber prepared by the scalable wet-spinning method is schematically illustrated in Fig. 1b (see details in the Experimental section). Briefly, the fibers are produced through coagulation of Mo1.33C/PEDOT:PSS paste in 1% polyethylenimine (PEI) solution where PEI with a rich ammonium end group, can act as a cross-linking polymer binding Mo1.33C and PEDOT:PSS to create a three-dimensional architecture within the fiber via self-assembling. To confirm that PEI, as a cross-linking agent, is involved in the self-assembly process of the fibers, FT-IR is performed as shown in Fig. 1e. The peak at 3310 cm−1 for PEI is produced by N–H vibrations of the terminal primary amine. This peak is retained in the fiber, which is direct evidence of PEI being involved in the fiber assembly. This method of cross-linker enable the fibers to exhibit high tensile strength (Fig. S2†). Scanning electron microscope (SEM) images show that the fiber has a porous relatively smooth outer wall that keeps the cylindrical structure from collapsing during the spinning process (Fig. 1f). The cross-section SEM images (Fig. 1g and h) of the fibers reveal that the 3D porous microstructure of the fibers is achieved by homogeneously dispersed Mo1.33C sheets and PEDOT:PSS. The Mo1.33C/PEDOT:PSS fiber exhibited a micropore-dominated pore-size distribution, consistent with the result of N2 adsorption–desorption (Fig. S3†). Within the hybrid fiber, the Mo1.33C, which have a large surface area, offers a high capacitance and electrical conductivity, while the laminated i-MXene/polymer structure and 3D network architecture offers more active sites and ion transport channels. These synergistic effects contribute to the enhanced electrochemical performance achieved in the hybrid fibers.The application of as-synthesized fibers in a capacitor was first studied in a three-electrode cell with a 3 M H2SO4 electrolyte. The pristine Mo1.33C film and PEDOT:PSS fibers were also investigated for reference (Fig. S4†). The cyclic voltammetry (CV) curves of the hybrid fibers for scan rates in the range 2–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mV s−1 revealed negligible distortion. As shown in Fig. 2a, the CV curves at low scan rate show reversible redox peaks at −0.3 to −0.2 V vs. Ag/AgCl. In addition, the small redox peaks in the range of −0.2 to −0.1 V (vs. Ag/AgCl) likely originates from traces of MoO3. Even for a scan rate up to 10
000 mV s−1 revealed negligible distortion. As shown in Fig. 2a, the CV curves at low scan rate show reversible redox peaks at −0.3 to −0.2 V vs. Ag/AgCl. In addition, the small redox peaks in the range of −0.2 to −0.1 V (vs. Ag/AgCl) likely originates from traces of MoO3. Even for a scan rate up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mV s−1, the pair of redox peaks persists, indicating excellent rate performance. To explore the charge storage kinetics, the relationship of cathodic and anodic peak current density against the scan rate is shown in Fig. 2b. The b-value obtained from the power-law (see ESI†) can directly reflect the charge storage kinetics. Herein, the fiber electrode exhibits predominantly surface reactions processes since the b value is close to 1 for a scan rate between 2 and 1000 mV s−1. This can be understood by the 3D network architecture of the fiber significantly reducing the ion diffusion distance. In addition, the galvanostatic charge/discharge (GCD) curves of the fiber electrodes at different current densities are highly symmetrical with a low iR drop (∼0.08 V) even at a high discharge current density of 30 A g−1, which confirm the high reversibility of the redox reactions and good Coulomb efficiency (Fig. 2c). The rate performance of the fiber electrodes is presented in Fig. 2d. The specific capacitance of the fiber electrodes decreased only slightly from 457 F g−1 to 309 F g−1 when the scan rate was increased from 2 to 10
000 mV s−1, the pair of redox peaks persists, indicating excellent rate performance. To explore the charge storage kinetics, the relationship of cathodic and anodic peak current density against the scan rate is shown in Fig. 2b. The b-value obtained from the power-law (see ESI†) can directly reflect the charge storage kinetics. Herein, the fiber electrode exhibits predominantly surface reactions processes since the b value is close to 1 for a scan rate between 2 and 1000 mV s−1. This can be understood by the 3D network architecture of the fiber significantly reducing the ion diffusion distance. In addition, the galvanostatic charge/discharge (GCD) curves of the fiber electrodes at different current densities are highly symmetrical with a low iR drop (∼0.08 V) even at a high discharge current density of 30 A g−1, which confirm the high reversibility of the redox reactions and good Coulomb efficiency (Fig. 2c). The rate performance of the fiber electrodes is presented in Fig. 2d. The specific capacitance of the fiber electrodes decreased only slightly from 457 F g−1 to 309 F g−1 when the scan rate was increased from 2 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mV s−1 (retention of 67.6%), the capacitance and retention of hybrid fiber electrode is surpasses the reported graphene, CNT or Ti3T2Tz based fibers (Table S1†).39,40 The excellent rate performance is consistent with the CV results, which can also be confirmed by electrochemical impedance spectroscopy (EIS, Fig. S5†).
000 mV s−1 (retention of 67.6%), the capacitance and retention of hybrid fiber electrode is surpasses the reported graphene, CNT or Ti3T2Tz based fibers (Table S1†).39,40 The excellent rate performance is consistent with the CV results, which can also be confirmed by electrochemical impedance spectroscopy (EIS, Fig. S5†).
To further explore the potential of fibers for energy storage, a flexible solid-state FSC is fabricated. The performance of the FSC device is investigated through CV curves at different scan rate (Fig. 2e). Similar with the fiber electrodes, a pair of redox peaks appears in the CV curves of the FSC device at low scan rates, which indicates a low charge transfer resistance and good reversibility. However, the CV curves show serious distortion with the increase of the scan rate. This may be caused by incomplete ion migration in gel electrolyte based supercapacitor devices. Therefore, the redox peak disappeared when the scan rate increased up to 200 mV s−1. The FSC device shows a capacitance of 110 F g−1 at a scan rate of 2 mV s−1 and remain above 61.3 F g−1 even at scan rates up to 1000 mV s−1 (Fig. S6†). The FSC device not only exhibits superior rate performance but also shows excellent cycling stability. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, a capacitance retention of 87% and coulombic efficiency of 99% are achieved at a rate of 20 A g−1 (Fig. 2f).
000 cycles, a capacitance retention of 87% and coulombic efficiency of 99% are achieved at a rate of 20 A g−1 (Fig. 2f).
Encouraged by the excellent specific capacitance of the FSC described above, an asymmetric fiber type supercapacitor (AFSCs) was assembled using Mo1.33C/PEDOT:PSS hybrid fiber and rGO fiber (Fig. S7–S9†) as the cathode and anode (Fig. 3a), respectively, which can further improve the energy density by widening the operating voltage. Notably, the AFSC achieve an operating potential window up to 1.6 V, which yields higher energy density compared to corresponding symmetric devices. The CV curves of the AFSCs at different scan rates showed excellent rate performance (Fig. 3b), showing negligible degradation up to 200 mV s−1. The nearly symmetric GCD curves of the AFSC at different current density demonstrate fast ion and electron transfer kinetics, highly reversible redox processes, and high coulombic efficiency (Fig. 3c). The capacitance of the device reaches 105 F g−1 at a scan rate of 2 mV s−1 and maintains a capacitance of 54 F g−1 at a scan rate of 1000 mV s−1 (Fig. 3d). In addition, the AFSC demonstrates excellent cycling stability, and a capacitive retention of 94% is achieved after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles by GCD tests at a current of 10 A g−1 (Fig. 3e). The EIS pattern, with almost negligible increase at the high frequencies before and after the cycles, shows that the internal resistance of the fiber electrode and gel electrolyte hardly changes after cycling (Fig. 3e inset, Fig. S12†). This indicates that the 3D porous structure of the fiber electrode can effectively resist the influence of the electrode during the process of ion migration into and out of the electrode. At low frequency, a large slope is present in all fiber electrodes, which may be attributed to the ability of the 3D porous structure to maintain highly accessible surface before and after cycling. Furthermore, AFSCs are conveniently integrated in series and/or parallel configurations to increase the output voltage or total capacitance (Fig. S13†). The Ragone plots of the energy and power densities for the AFSCs are compared with those reported in the literatures.21,41–46 As shown in Fig. 3f, the fiber-based asymmetric device is superior to the symmetric device. A maximum energy density of 37 mW h g−1 is reached at a power density of 805 mW g−1. Notably, this energy density value is higher than those of recently reported fiber-based supercapacitors.43–45 The outstanding performance of the Mo1.33C/PEDOT:PSS fiber based supercapacitors originates from a high electrode conductivity, fast electrolyte diffusion kinetics provided by 3D network architecture, and a fast pseudocapacitive storage mechanism.
000 cycles by GCD tests at a current of 10 A g−1 (Fig. 3e). The EIS pattern, with almost negligible increase at the high frequencies before and after the cycles, shows that the internal resistance of the fiber electrode and gel electrolyte hardly changes after cycling (Fig. 3e inset, Fig. S12†). This indicates that the 3D porous structure of the fiber electrode can effectively resist the influence of the electrode during the process of ion migration into and out of the electrode. At low frequency, a large slope is present in all fiber electrodes, which may be attributed to the ability of the 3D porous structure to maintain highly accessible surface before and after cycling. Furthermore, AFSCs are conveniently integrated in series and/or parallel configurations to increase the output voltage or total capacitance (Fig. S13†). The Ragone plots of the energy and power densities for the AFSCs are compared with those reported in the literatures.21,41–46 As shown in Fig. 3f, the fiber-based asymmetric device is superior to the symmetric device. A maximum energy density of 37 mW h g−1 is reached at a power density of 805 mW g−1. Notably, this energy density value is higher than those of recently reported fiber-based supercapacitors.43–45 The outstanding performance of the Mo1.33C/PEDOT:PSS fiber based supercapacitors originates from a high electrode conductivity, fast electrolyte diffusion kinetics provided by 3D network architecture, and a fast pseudocapacitive storage mechanism.
Electrochemical transistors (ECTs) have attracted great attention for flexible electronics and bioelectronics applications ranging from printable logic circuits for e-textiles to drivers for sensors and neuromorphic devices.47–50 However, the channel components are mainly organic semiconductors or organic polyelectrolytes, while the research on inorganic semiconductor materials or organic-inorganic composite materials is sparse. In order to further expand the application field of Mo1.33C/PEDOT:PSS fibers, a fiber type ECT was fabricated by a simple parallel configuration (Fig. 4a). The two ends of the Mo1.33C/PEDOT:PSS fiber represented the ECT source and drain terminals, whereas the rGO fiber served as the gate electrode. Fig. 4 shows a typical set of output, transfer and transient curves of the fiber type ECT device. When a positive bias voltage is applied to the gate, the channel conductivity will decrease due to dedoping, and thus reduce the drain-source current (ID). As shown in Fig. 4b, the output characteristics of the device show saturation of ID with increasing drain-source voltage (VD) and operates at low gate voltages (VG < 1.0 V). At the same time, the device shows a large transconductance of 10.8 mS (Fig. 4c). The fiber type ECT shows a switching behavior from ON to OFF as the gate voltage pulses from VG = 0 to 1 V (Fig. 4d). The response time of this fiber type ECT is 0.1 and 0.03 s for the switch on and switch off, respectively. These values are better than that of planar ECT of PEDOT:PSS (Fig. S14†). Considering that ECT operation must rely on both electron and ion transport, all the results suggest that the Mo1.33C/PEDOT:PSS fiber have a good balance/optimization of these two processes, that is, the 3D composite network architecture has excellent electron and ion transport kinetics.
Conclusions
In summary, we demonstrate a scalable strategy to realize a multifunctional flexible i-MXene based smart fiber by spinning Mo1.33C/PEDOT:PSS hybrid paste from PEI solution, which is simple, efficient, and easily scaled up for mass production. The as-prepared i-MXene fibers, with high electrochemical activity and interconnected 3D network architecture, is beneficial for both electron and ion transport and storage. A solid-state, fiber-based asymmetric device was fabricated by using Mo1.33C/PEDOT:PSS fiber and rGO fiber, which can be used for both supercapacitors and electrochemical transistors, ECT. The asymmetric fiber type supercapacitor, AFSC, with an operating potential window of 1.6 V, showed a high energy density (37 mW h g−1), a high capacitance retention and cycling stability. The resulting fiber type ECT exhibits fast ion intercalation/de-intercalation, high transconductance and short response time. The here presented design strategies for multifunctional fiber devices show potential for applications in wearable and portable electronics.Author contributions
Leiqiang Qin and Jianxia Jiang conducted the materials synthesis and the structural and electrochemical characterization. Leiqiang Qin, and Johanna Rosen analyzed the experimental data. Leiqiang Qin, Fengling Zhang and Lintao Hou prepared the manuscript. Johanna Rosen supervised the project and co-wrote the manuscript. All the authors discussed the results and contributed to writing the manuscript.Conflicts of interest
The authors declare that they do not have any conflicts of interest.Acknowledgements
This work was financed by the SSF Synergy Program (EM16-0004), the Swedish Energy Agency (EM 42033-1) and by the Knut and Alice Wallenberg (KAW) Foundation through a Fellowship Grant and a Project Grant (KAW2020.0033). Support from the National Natural Science Foundation of China (61774077, 52103212), the Youth Projects of Joint Fund of Basic and Applied Basic Research Fund of Guangdong Province (2020A1515110738), the Key Projects of Joint Fund of Basic and Applied Basic Research Fund of Guangdong Province (2019B1515120073), the High-End Foreign Experts Project (G20200019046) and the Guangzhou Key laboratory of Vacuum Coating Technologies and New Energy Materials Open Projects Fund (KFVE20200006) is also acknowledged.References
- J. Liu, M. A. G. Namboothiry and D. L. Carroll, Appl. Phys. Lett., 2007, 90, 063501 CrossRef.
- B. O'Connor, K. P. Pipe and M. Shtein, Appl. Phys. Lett., 2008, 92, 193306 CrossRef.
- M. R. Lee, R. D. Eckert, K. Forberich, G. Dennler, C. J. Brabec and R. A. Gaudiana, Science, 2009, 324, 232–235 CrossRef CAS PubMed.
- J. Pu, K. Zhang, Z. Wang, C. Li, K. Zhu, Y. Yao and G. Hong, Adv. Funct. Mater., 2021, 31, 2106315 CrossRef CAS.
- L. Li, Q. Zhang, B. He, R. Pan, Z. Wang, M. Chen, Z. Wang, K. Yin, Y. Yao, L. Wei and L. Sun, Adv. Mater., 2022, 34, 2104327 CrossRef CAS PubMed.
- M. Maccioni, E. Orgiu, P. Cosseddu, S. Locci and A. Bonfiglio, Appl. Phys. Lett., 2006, 89, 143515 CrossRef.
- Q. Zhang, L. Li, H. Li, L. Tang, B. He, C. Li, Z. Pan, Z. Zhou, Q. Li, J. Sun, L. Wei, X. Fan, T. Zhang and Y. Yao, Nano Energy, 2019, 60, 267–274 CrossRef.
- M. Hamedi, L. Herlogsson, X. Crispin, R. Marcilla, M. Berggren and O. Inganäs, Adv. Mater., 2009, 21, 573–577 CrossRef CAS PubMed.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- R. Mas-Balleste, C. Gomez-Navarro, J. Gomez-Herrero and F. Zamora, Nanoscale, 2011, 3, 20–30 RSC.
- J. Liu, H.-B. Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z.-Z. Yu, Adv. Mater., 2017, 29, 1702364 Search PubMed.
- N. Kurra, B. Ahmed, Y. Gogotsi and H. N. Alshareef, Adv. Energy Mater., 2016, 6, 1601372 CrossRef.
- Z. Dong, C. Jiang, H. Chen, Y. Zhao, G. Shi, L. Jiang and L. Qu, Adv. Mater., 2012, 24, 1856–1861 CrossRef CAS PubMed.
- A. B. Dalton, S. Collins, E. Munoz, J. M. Razal, V. H. Ebron, J. P. Ferrais, J. N. Coleman, B. G. Kim and R. H. Baughman, Nature, 2003, 423, 703 CrossRef CAS PubMed.
- X. Chen, L. Qiu, J. Ren, G. Guan, H. Lin, Z. Zhang, P. Chen, Y. Wang and H. Peng, Adv. Mater., 2013, 25, 6436–6441 CrossRef CAS PubMed.
- Z. Zhou, W. Panatdasirisuk, T. S. Mathis, B. Anasori, C. Lu, X. Zhang, Z. Liao, Y. Gogotsi and S. Yang, Nanoscale, 2018, 10, 6005–6013 RSC.
- L. Kou, T. Huang, B. Zheng, Y. Han, X. Zhao, K. Gopalsamy, H. Sun and C. Gao, Nat. Commun., 2014, 5, 3754 CrossRef CAS PubMed.
- S. H. Aboutalebi, R. Jalili, D. Esrafilzadeh, M. Salari, Z. Gholamvand, S. A. Yamini, K. Konstantinov, R. L. Shepherd, J. Chen, S. E. Moulton, P. C. Innis, A. I. Minett, J. M. Razal and G. G. Wallace, ACS Nano, 2014, 8, 2456–2466 CrossRef CAS PubMed.
- L. Liu, Y. Yu, C. Yan, K. Li and Z. Zheng, Nat. Commun., 2015, 6, 7260 CrossRef CAS PubMed.
- Y. Meng, Y. Zhao, C. Hu, H. Cheng, Y. Hu, Z. Zhang, G. Shi and L. Qu, Adv. Mater., 2013, 25, 2326–2331 CrossRef CAS PubMed.
- S. Seyedin, E. R. S. Yanza and J. M. Razal, J. Mater. Chem. A, 2017, 5, 24076–24082 RSC.
- G. Sun, X. Zhang, R. Lin, J. Yang, H. Zhang and P. Chen, Angew. Chem., Int. Ed., 2015, 54, 4651–4656 CrossRef CAS PubMed.
- N. Yu, H. Yin, W. Zhang, Y. Liu, Z. Tang and M.-Q. Zhu, Adv. Energy Mater., 2016, 6, 1501458 CrossRef.
- J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Liang, D. Chen and G. Shen, ACS Nano, 2013, 7, 5453–5462 CrossRef CAS PubMed.
- S. Zhai, C. Wang, H. E. Karahan, Y. Wang, X. Chen, X. Sui, Q. Huang, X. Liao, X. Wang and Y. Chen, Small, 2018, 14, 1800582 CrossRef PubMed.
- Q. Jiang, N. Kurra, M. Alhabeb, Y. Gogotsi and H. N. Alshareef, Adv. Energy Mater., 2018, 8, 1703043 CrossRef.
- X. Yang, Q. Wang, K. Zhu, K. Ye, G. Wang, D. Cao and J. Yan, Adv. Funct. Mater., 2021, 31, 2101087 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- L. Qin, J. Jiang, Q. Tao, C. Wang, I. Persson, M. Fahlman, P. O. Å. Persson, L. Hou, J. Rosen and F. Zhang, J. Mater. Chem. A, 2020, 8, 5467–5475 RSC.
- J. Chen, M. Chen, W. Zhou, X. Xu, B. Liu, W. Zhang and C. Wong, ACS Nano, 2022, 16, 2461–2470 CrossRef CAS PubMed.
- X. Yang, Y. Yao, Q. Wang, K. Zhu, K. Ye, G. Wang, D. Cao and J. Yan, Adv. Funct. Mater., 2022, 32, 2109479 CrossRef CAS.
- J. Jiang, L. Qin, J. Halim, P. O. A. Persson, L. Hou and J. Rosen, Nano Res., 2021, 15, 3587–3593 CrossRef.
- A. VahidMohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, 6547 CrossRef PubMed.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1332 CrossRef CAS PubMed.
- M. Naguib, J. Halim, J. Lu, K. M. Cook, L. Hultman, Y. Gogotsi and M. W. Barsoum, J. Am. Chem. Soc., 2013, 135, 15966–15969 CrossRef CAS PubMed.
- Q. Tao, M. Dahlqvist, J. Lu, S. Kota, R. Meshkian, J. Halim, J. Palisaitis, L. Hultman, M. W. Barsoum, P. O. Å. Persson and J. Rosen, Nat. Commun., 2017, 8, 14949 CrossRef PubMed.
- L. Qin, Q. Tao, A. Ghazaly, J. Fernández-Rodríguez, P. O. Å. Persson, J. Rosen and F. Zhang, Adv. Funct. Mater., 2018, 28, 1703808 CrossRef.
- B. Ahmed, A. Ghazaly and J. Rosen, Adv. Funct. Mater., 2020, 30, 2000894 CrossRef CAS.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- Z. Bo, W. Zhu, W. Ma, Z. Wen, X. Shuai, J. Chen, J. Yan, Z. Wang, K. Cen and X. Feng, Adv. Mater., 2013, 25, 5799–5806 CrossRef CAS PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- P. Li, Z. Jin, L. Peng, F. Zhao, D. Xiao, Y. Jin and G. Yu, Adv. Mater., 2018, 30, 1800124 CrossRef PubMed.
- C. Zhou, T. Gao, Y. Wang, Q. Liu, Z. Huang, X. Liu, M. Qing and D. Xiao, Small, 2019, 15, 1803469 CrossRef PubMed.
- T. Ni, S. Wang, J. Shi, X. Du, Q. Cheng, Z. Dong, L. Ruan, W. Zeng, X. Guo, X. Ren and Z. Huang, Adv. Mater. Technol., 2020, 5, 2000268 CrossRef CAS.
- X. Zheng, L. Yao, Y. Qiu, S. Wang and K. Zhang, ACS Appl. Energy Mater., 2019, 2, 4335–4344 CrossRef CAS.
- J. Zhang, S. Seyedin, S. Qin, Z. Wang, S. Moradi, F. Yang, P. A. Lynch, W. Yang, J. Liu, X. Wang and J. M. Razal, Small, 2019, 15, 1804732 CrossRef PubMed.
- Z. Wang, S. Qin, S. Seyedin, J. Zhang, J. Wang, A. Levitt, N. Li, C. Haines, R. Ovalle-Robles, W. Lei, Y. Gogotsi, R. H. Baughman and J. M. Razal, Small, 2018, 14, 1802225 CrossRef PubMed.
- S. Fabiano, N. Sani, J. Kawahara, L. Kergoat, J. Nissa, I. Engquist, X. Crispin and M. Berggren, Sci. Adv., 2017, 3, e1700345 CrossRef PubMed.
- Y. van de Burgt, E. Lubberman, E. J. Fuller, S. T. Keene, G. C. Faria, S. Agarwal, M. J. Marinella, A. A. Talin and A. Salleo, Nat. Mater., 2017, 16, 414–418 CrossRef CAS PubMed.
- P. Gkoupidenis, D. A. Koutsouras and G. G. Malliaras, Nat. Commun., 2017, 8, 15448 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta01428a |
| ‡ Leiqiang Qin and Jianxia Jiang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |