Bio-inspired ultra-thin microfluidics for soft sweat-activated batteries and skin electronics†
Abstract
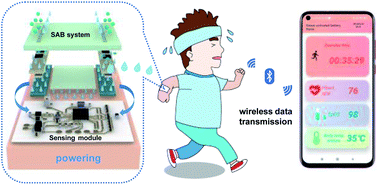
Thin, soft, skin electronics have attracted great attention for their use in the fields of healthcare monitoring and human–machine interfaces. The power supply is always one of the key issues in skin electronics, as it provides electrical energy to sensors, wireless communication modules, chips, and so on. However, traditional batteries generally have limitations such as rigid formats, large volume, or even potential safety issues by hazard materials, which might not be applicable for skin electronics. Herein, we present an ultra-thin, soft and biocompatible sweat-activated battery, combining bio-inspired microfluidics with flexible electronic technologies. The sweat-activated battery can collect the human sweat in seconds and yield a power density up to 122 mW cm−2 and a discharge capacity of 8.33 mA h. The sweat-activated battery can provide sufficient power to drive the state-of-the-art wearable electronics for real-time monitoring physiological signals (including heart rate, blood oxygen and skin temperature) and wireless transmission to smartphones via Bluetooth.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...