Radical-mediated C–N bond activation in 3,5-diamino-4-nitro-1H-pyrazole towards high-energy and insensitive energetic materials†
Abstract
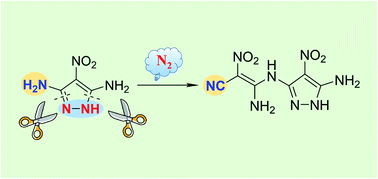
C–N bond activation has been recognized as a powerful methodology in organic synthesis, but C–N bond activation on monocyclic rings remains an intractable challenge, due to the high energy barrier required for the dearomatization process. Now a facile and efficient nitrogen-centered-radical-mediated approach to cleave C–N bonds in a monocyclic pyrazole is described. Using N-bromosuccinimide as a radical initiator, C–N bond cleavage was achieved in yields up to 91%. This reaction led to an important precursor (2), subsequently annulated and oxidized to an energetic compound 4, which exhibits promising application in balancing performances and thermal stabilities.


 Please wait while we load your content...
Please wait while we load your content...