Hierarchical trace copper incorporation activated cobalt layered double hydroxide as a highly selective methanol conversion electrocatalyst to realize energy-matched photovoltaic-electrocatalytic formate and hydrogen co-production†
Abstract
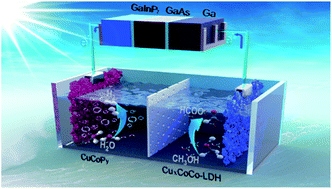
Novel hierarchical copper cobalt layered double hydroxide (CuxCoCo-LDH) nanosheet networked with tunable stoichiometry are designed and synthesized for the first time by in situ pseudomorphic transformation of heterometallic CuxCo-MOFs as electrocatalysts for the methanol-oxidation-to-formate reaction (MOFR). The incorporation of trace Cu with a strong Jahn–Teller effect induces collective impacts on the morphology and electronic structure, leading to a superior MOFR performance (i.e., an overpotential of only 50 mV at 10 mA cm−2, and a faradaic efficiency of nearly 100% for formate production). Density functional theory (DFT) reveals that Cu incorporation initiates lattice distortion and charge redistribution and further finely tunes the surface adsorption properties of the catalyst for reactants and intermediates (i.e., Cu doping enhances the adsorption of methanol and weakens the adsorption of formic acid on the catalyst surface). Based on the successful synthesis of high-efficiency MOFR electrocatalysts, we present a valuable approach to optimize the electrolysis voltage through replacing the conventional anodic water oxidation with the thermodynamically more favorable MOFR, and thus realize the energy match between the photovoltaic cell and electrolysis cell, further achieving spontaneous and efficient co-generation of formate and hydrogen.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...