Unraveling the role of solvent–precursor interaction in fabricating heteroatomic carbon cathode for high-energy-density Zn-ion storage†
Abstract
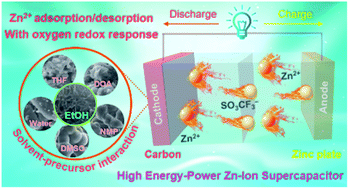
Adsorption-type carbon cathodes deliver great potential for application in aqueous Zn-based hybrid supercapacitors but systematic tailoring of carbon microstructures to optimize the supercapacitive activity of the Zn ion remains a perplexing topic. Herein, a solvent-mediated strategy has been demonstrated to design heteroatomic carbons with tailor-made features for activating Zn storage sites. The solvent–precursor interaction is optimized by a solubility parameter model and molecular growth trajectory simulations, which promote the thermodynamic solubilization (−5.93 kcal mol−1) and growth kinetics (−151.4 kcal mol−1) of the polymeric intermediates with an energy-minimized reaction roadblock. Flake-shaped carbon nanoarchitectures with substantial electrosorption spaces (1022 m2 g−1) and rich O/N motifs (6.03/6.01 wt%) are also demonstrated. Such profitable features implanted in the customized nanocarbon enable low ion migration hurdles for accommodating electroactive species and fast heteroatomic phase conversion for delivering redox response, contributing to a super-high energy density of 92.8 W h kg(cathode)−1 and an ultrastable cycle-life of 40 000 cycles at 40 A g−1 in the constructed Zn-based hybrid supercapacitor. Comprehensive characterization untangles the high-kinetics chemical adsorption of N-substituted active sites and the powerful redox reactivity of the hydroxyl/carboxyl functionalities on the host Zn ions during each round-trip discharge/charge cycle. This work proposes a design concept of regulating the solvent–precursor interaction for structuring carbons toward highly efficient Zn-ion storage.
- This article is part of the themed collections: 2023 Journal of Materials Chemistry A Lunar New Year collection and 2022 Journal of Materials Chemistry A Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...