Structure, composition and electrochemical performance analysis of fluorophosphates from different synthetic methods: is really Na3V2(PO4)2F3 synthesized?†
Abstract
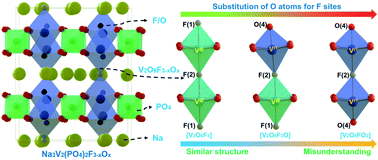
Sodium vanadium fluorophosphate, Na3V2(PO4)2F3 (NVPF), has been confirmed to be a potential cathode material for commercial sodium-ion batteries, owing to its high operating voltage and super ionic conductivity. However, different charge/discharge potentials were reported in many “pure phase NVPF” research articles. In this work, we tried to clarify the misunderstandings about the NVPF composition and structure with comprehensive investigation by sol–gel, solid-state and hydrothermal methods. Relatively pure NVPF obtained by the solid-state method gradually decomposes into Na3V2(PO4)3 above 600 °C. The sol–gel method would introduce O atoms into the F sites of the NVPF crystal structure, forming Na3V2(PO4)2F3−yOz and leading to a higher decomposition temperature. Na3(VO)2(PO4)2F single crystals produced by the hydrothermal method exhibit the best structural stability. Furthermore, the better crystallinity indicates higher specific capacity and cycle performances in all experiments. These novel findings reveal the relationship among F loss, structural changes, and electrochemical properties and provide a new understanding for the synthesis of NVPF cathodes.


 Please wait while we load your content...
Please wait while we load your content...