A unique 2.1 V “Water in Salt” elemental sulfur based Na-ion hybrid storage capacitor†
Abstract
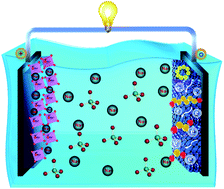
The aqueous sodium-ion hybrid capacitor is a novel energy storage device that reconciles the high energy and power density in a single device along with the inherent safety and conductivity of an aqueous electrolyte. However, the low capacity of electrode materials, low water stability window, and kinetic incongruity between the two types of materials present a daunting challenge that has hindered its widespread application. Herein we have manifested a new elemental sulfur based Na-ion/S hybrid storage capacitor featuring mesoporous nitrogen-containing carbon (MNC) anchoring sulfur as a negative and Na0.44MnO2 as a positive electrode material in “Water in Salt” Electrolyte (WiSE). The resultant device delivers an output voltage of 2.1 V with an impressive energy density of 84.33 W h kg−1 and a maximum power density of 6.683 kW kg−1 with a remarkable 81.5% capacitance retention after 5000 cycles at 10 A g−1. Besides, for the first time, an entirely paper-based Na-ion/S hybrid storage capacitor was constructed that demonstrates high flexibility and energy output under extreme conditions of twisting and bending, and even after punching holes. These results suggest a new high-performance energy storage device with great potential for practical application.


 Please wait while we load your content...
Please wait while we load your content...